1. Introduction to Electron Configuration
Understanding the what’s the electron configuration for carbon is vital for grasping the fundamentals of chemistry and atomic theory. Electron configuration is a representation of how electrons are distributed in an atom’s electron shells and orbitals. This concept not only helps in explaining the chemical properties of elements but also illuminates how atoms interact to form compounds.
1.1 What is Electron Configuration?
Electron configuration describes the arrangement of electrons in an atom. Electrons occupy orbitals in a manner that minimizes energy, obeying principles such as the Pauli Exclusion Principle, Hund’s Rule, and the Aufbau Principle. The configuration is typically expressed in the form of detailed notation, such as ‘1s² 2s² 2p²’ for carbon, indicating the number of electrons in each subshell.
1.2 Importance of Electron Configuration in Chemistry
Electron configuration plays a critical role in predicting chemical behavior. It determines an atom’s reactivity, the types of bonds it can form, and its overall placement in the periodic table. For instance, the number of valence electrons ultimately dictates an element’s interactions with others, thus influencing molecular structure and reactivity patterns.
1.3 Overview of Carbon’s Role in Chemistry
Carbon stands out in the periodic table due to its unique electron configuration, making it capable of forming a diverse array of compounds. With the atomic number 6, carbon serves as a backbone for organic molecules, participating in single and double bonds, hybridization, and various electronic encounters in chemical reactions. Its ability to bond with multiple elements—including itself—allows for the complexity of organic chemistry and life as we know it.
2. The Basics of Carbon’s Electron Configuration
2.1 Atomic Structure Overview
To fully comprehend carbon’s electron configuration, one must understand the underlying atomic structure. Each element is defined by its atomic number—the number of protons within the nucleus. For carbon, this number is six, which corresponds to six electrons in a neutral state. These electrons are arranged in energy levels or shells around the nucleus, where the first shell can hold two electrons, and subsequent shells can hold more, starting with eight.
2.2 The Electron Configuration of Carbon Explained
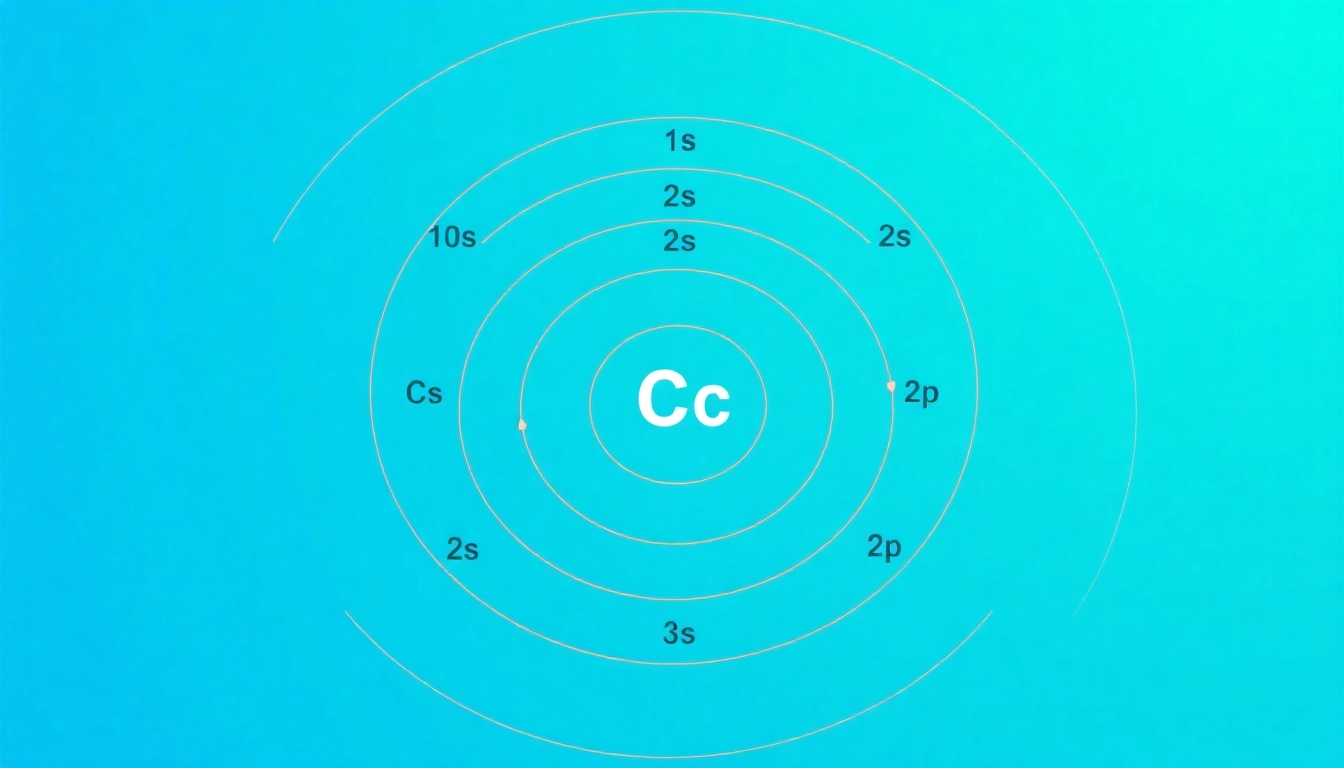
The electron configuration of carbon can be specifically understood as follows: 1s² 2s² 2p². Here, the ‘1s’ shell contains two electrons, filling it to capacity, while ‘2s’ also holds two electrons. The final group, ‘2p²’, signifies that carbon has two electrons in the 2p orbital, which holds a maximum of six. This configuration highlights that carbon has four valence electrons—two in the s orbital and two in the p orbital, vital for bonding and reactivity.
2.3 How to Determine Electron Configurations
To determine the electron configuration for any atom, one follows a sequential filling according to Hund’s Rule and the Aufbau Principle. The process includes determining the atomic number and filling the orbitals from the lowest energy level to the highest. The configuration can also be influenced by the presence of unpaired electrons when creating molecular bonds.
3. Writing the Electron Configuration for Carbon
3.1 Steps to Write the Configuration
Writing the electron configuration involves several logical steps:
- Identify the atomic number: Carbon’s atomic number is 6.
- Fill orbitals in order of increasing energy levels, starting from the lowest: 1s < 2s < 2p.
- Account for electron capacity in each orbital and shell until the total number of electrons equals the atomic number.
- Write down the configuration: Carbon results in 1s² 2s² 2p².
3.2 Common Notation Practices
There are several notation practices in representing electron configurations, including:
- Standard Notation: This is the common method (e.g., 1s² 2s² 2p²).
- Condensed Notation: Uses noble gas symbols to condense the electron configuration (e.g., [He] 2s² 2p² for carbon).
- Orbital Diagrams: Visual representations showing the filling order of orbitals, illustrating the pairing of electrons and unpaired states.
3.3 Visualizing Carbon’s Electron Configuration
Visual aids such as orbital diagrams and electron configuration charts can help better understand the concept of carbon’s electron arrangement. These diagrams display the filling order and help illustrate how electrons are paired within an orbital, which is crucial for predicting chemical reactivity and bonding patterns.
4. Variations in Writing Electron Configuration
4.1 Differences in Notation: Line vs. Orbital Diagrams
While line notation focuses on writing out the electron configuration, orbital diagrams can offer immediate visual representation. Both methods serve their purposes: line notation is succinct for documentation, while orbital diagrams allow for understanding electron pairing and unpaired electrons, which are critical in bonding considerations.
4.2 Electron Configuration for Carbon Ions
When carbon adopts different ionic states, its electron configuration changes. For example, a cation of carbon (C4+) would lose four electrons, resulting in the configuration of 1s². Conversely, an anion such as C4- gains four electrons, leading to the configuration of 1s² 2s² 2p⁶, which is an octet similar to neon.
4.3 Misconceptions about Carbon’s Electron Configuration
Several misinterpretations exist regarding carbon’s electron configurations. A common mistake is overlooking that each element’s configuration is unique to its electron count and arrangement. Some may confuse the standard configurations with excited states where electrons could jump to higher orbitals. Understanding that energy levels and their capacities are fundamental can help eliminate these misconceptions.
5. Practical Applications of Carbon’s Electron Configuration
5.1 Understanding Bonding and Molecular Structure
Carbon’s electron configuration directly influences its ability to form covalent bonds. The four valence electrons enable carbon to bond with various elements—forming single, double, and even triple bonds, making it a cornerstone of organic chemistry. Concepts such as hybridization, where atomic orbitals mix to form new hybrid orbitals for bonding, arise from the examination of electron configurations.
5.2 The Role of Carbon in Organic Chemistry
Being the primary building block of organic compounds, carbon’s electron configuration facilitates the formation of diverse molecular structures. Its tetravalent nature, arising from having four valence electrons, allows for complex arrangements in various organic compounds, leading to the vast diversity found in biological systems and synthetic materials.
5.3 Advanced Topics: Hybridization and Reactivity of Carbon
Advanced exploration of carbon’s electron configuration reveals insights into hybridization, where carbon atoms form new orbitals (sp3, sp2, and sp) to enable various bonding geometries. This concept elucidates reactivity and stability across myriad carbon compounds, explaining how configurations influence properties such as boiling points, melting points, and solubility. Understanding these interactions is crucial in fields such as material science, biochemistry, and pharmacology.