1. Introduction to Electron Configuration
1.1 What Is Electron Configuration?
Electron configuration refers to the distribution of electrons in an atom’s orbitals. Each element has a unique electron configuration that determines its chemical behavior and interactions. The configuration is expressed in a notation that indicates the number of electrons in each subshell or orbital, showcasing how electrons are organized around the nucleus.
1.2 Importance of Electron Configurations in Chemistry
Understanding electron configurations is crucial for grasping various chemical principles. They play a vital role in predicting the reactivity, bonding characteristics, and the physical properties of elements. Moreover, they are foundational to areas such as organic chemistry, materials science, and the development of new chemical substances.
1.3 Overview of Carbon’s Role in the Periodic Table
Carbon is a fundamental component of life on Earth and is known for its versatility in forming compounds. With an atomic number of 6, it is located in group 14 (IVA) of the periodic table. Carbon’s electron configuration is pivotal to its ability to bond with other elements, forming a diverse array of organic and inorganic compounds. For a detailed description of carbon’s electron configuration, you can visit what does the electron configuration of carbon look like?
2. The Basics of Carbon Atom Structure
2.1 Atomic Number and Weight of Carbon
Carbon has an atomic number of 6, which means it has six protons in its nucleus. Its atomic weight is approximately 12.011 u (atomic mass units), considering the most abundant isotopes, primarily carbon-12 (with 6 neutrons) and carbon-13 (with 7 neutrons). This unique composition enables carbon to form a vast range of molecules essential for life.
2.2 The Concept of Orbitals and Energy Levels
Carbon’s electrons are arranged in specific energy levels, known as shells, and further divided into orbitals within these shells. The first energy level can hold a maximum of 2 electrons in the 1s orbital, while the second energy level can accommodate up to 8 electrons across the 2s and 2p orbitals. The arrangement of these electrons across these energy levels informs us as to how carbon interacts with other elements.
2.3 The Neutron and Proton Composition
A neutral carbon atom contains 6 protons and 6 electrons, with the neutrons determining the isotope of carbon. The most stable isotope, carbon-12, contains 6 neutrons, while carbon-14, known for its role in dating organic materials, has 8 neutrons. This composition is critical for understanding both nuclear properties and chemical reactivity.
3. Detailed Look at Carbon’s Electron Configuration
3.1 Step-by-Step Breakdown: Writing the Configuration
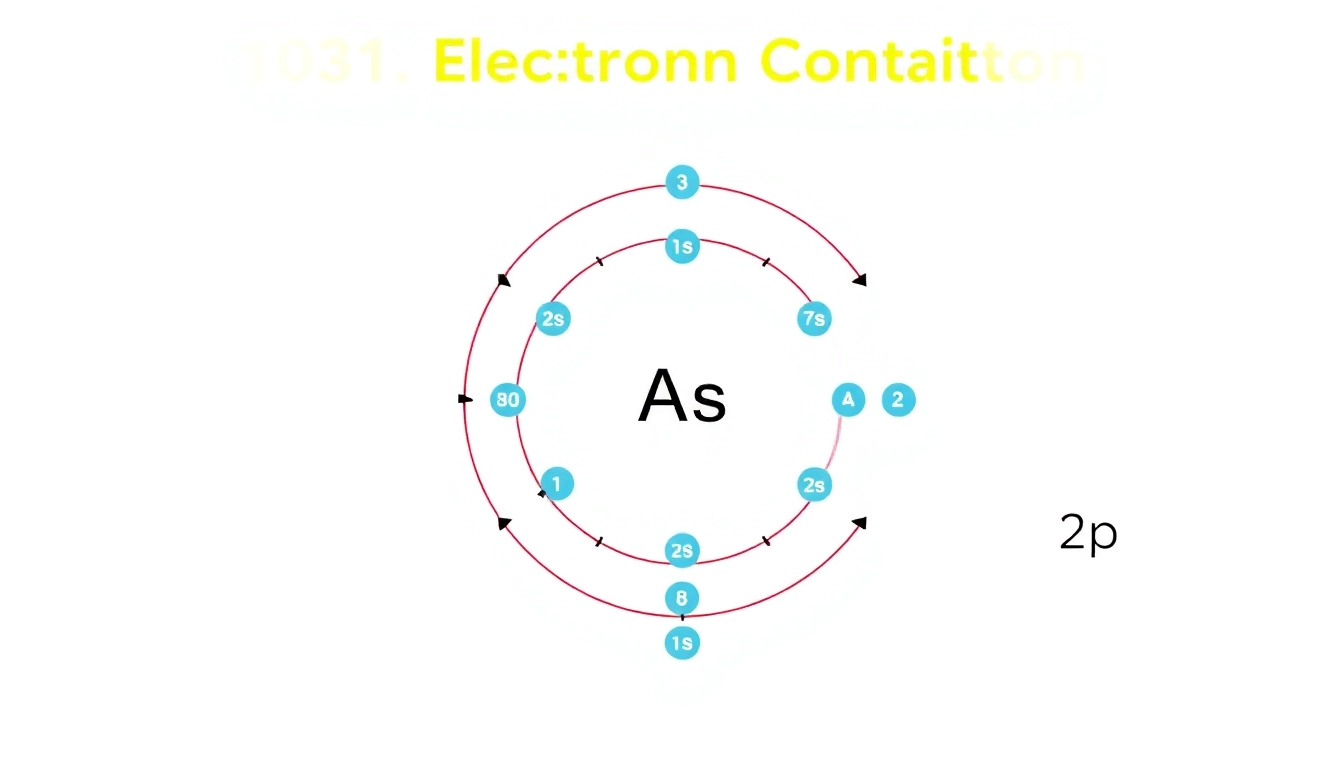
The electron configuration of carbon is represented as 1s2 2s2 2p2. This notation signifies that:
- 2 electrons occupy the 1s orbital (closest to the nucleus).
- 2 electrons are present in the 2s orbital, indicating a filled subshell.
- 2 electrons occupy the 2p orbitals, which can hold up to 6 electrons total.
This distribution illustrates carbon’s position as a tetravalent element, meaning it can form four covalent bonds with other atoms.
3.2 Visual Representation of 1s, 2s, and 2p Orbitals
A visual representation can greatly aid in understanding how these orbitals are structured. The 1s orbital is spherical and closely surrounds the nucleus, while the 2s orbital is also spherical but larger. The 2p orbitals are shaped like dumbbells and are oriented along the x, y, and z axes in space, allowing for various bonding orientations.
3.3 Common Misconceptions and Clarifications
One common misconception is that all electrons are evenly distributed among orbitals. In reality, electrons occupy the lowest energy levels first, as per the Aufbau principle. Additionally, Hund’s rule states that electrons will fill degenerate orbitals singly before pairing up. Understanding these rules is essential for accurately predicting chemical behavior and bonding capabilities.
4. Applications of Carbon’s Electron Configuration
4.1 Why Carbon’s Configuration Matters in Organic Chemistry
Carbon’s unique electron configuration and its ability to form four covalent bonds enable it to act as a backbone for many organic compounds. This tetravalency allows carbon to create chains, rings, and branching structures, which leads to the vast diversity of organic chemistry, essential in biochemistry, pharmaceuticals, and materials science.
4.2 Impact on Chemical Bonding and Molecular Structure
The electron configuration of carbon influences how it bonds with other elements, impacting molecular geometry and stability. For example, carbon can form single, double, or triple bonds with various elements, affecting molecular shapes and reactivity. Understanding these concepts helps chemists design novel compounds with specific characteristics.
4.3 Related Elements and Their Configurations
Other elements in the same group as carbon, such as silicon and germanium, have similar electron configurations and exhibit analogous properties in terms of bonding and molecular structure. These elements are vital in industries ranging from semiconductors to polymer science, illustrating the broader implications of electron configuration beyond carbon itself.
5. Conclusion and Further Reading
5.1 Summary of Key Takeaways
In summary, the electron configuration of carbon, 1s2 2s2 2p2, plays a pivotal role in determining its chemical properties and reactivity. Understanding this configuration enables chemists to predict behavior and interactions accurately, demonstrating carbon’s unique status in the periodic table.
5.2 Recommended Resources for Deepening Understanding
For those looking to further expand their knowledge on electron configurations, numerous resources are available, including advanced chemistry textbooks, reputable online courses, and educational videos. Engaging with these materials can provide deeper insights into chemical principles and applications.
5.3 Encouragement to Explore More about Electron Configurations
As the study of electron configuration is integral to both theoretical and practical chemistry, continuing exploration in this field can lead to significant discoveries and innovations. Whether one is a student, educator, or professional chemist, the impact of electron configurations is profound and warrants ongoing inquiry and research.