1. Introduction to Carbon and Its Electrons
Carbon is one of the most fundamental building blocks of life, playing a crucial role in the composition of organic molecules. It is the sixth element on the periodic table, represented by the symbol ‘C’. Understanding carbon’s electrons is fundamental to grasping its chemistry and its importance in biological processes. From the fuels that power our lifestyles to the structures that sustain life, carbon’s properties are intricately tied to its electronic structure. In this comprehensive guide, we’ll delve into the details of carbon’s electrons, including concepts such as electron configuration and bonding.
1.1 Overview of Carbon’s Atomic Structure
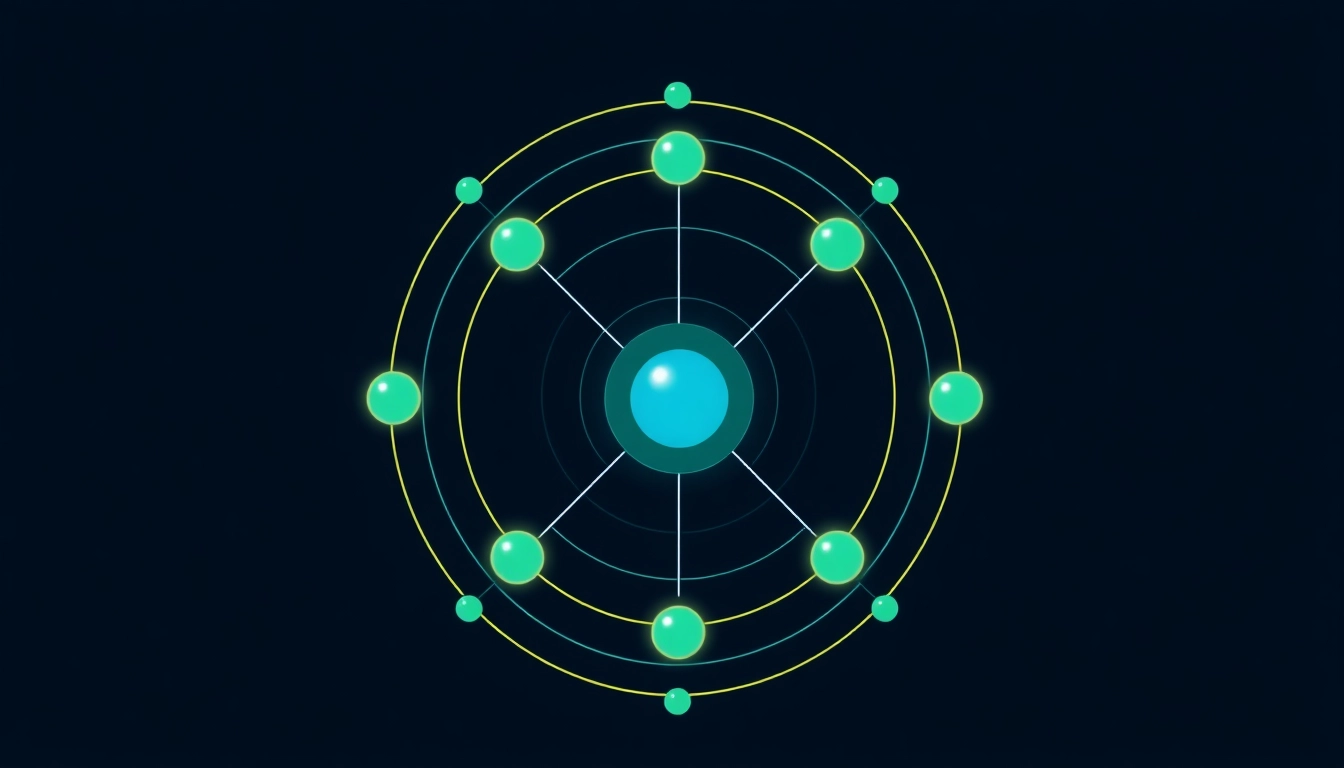
Every carbon atom consists of six protons, six neutrons, and six electrons, which correspond to its atomic number of 6. The protons and neutrons are located in the atom’s nucleus, while the electrons occupy various energy levels, also known as electron shells. These shells are critical for dictating how carbon interacts with other elements. The first shell can hold a maximum of two electrons, and the second shell can hold a maximum of eight. For carbon, the distribution of these electrons is notably significant in determining its chemical behavior and valency.
1.2 Importance of Electrons in Chemical Reactions
Electrons are essential in facilitating chemical reactions. They are the particles that form bonds with other atoms, resulting in the creation of molecules. In carbon, the arrangement of its electrons particularly impacts its ability to form four covalent bonds, making it a versatile element in organic chemistry. This adaptability is one of the reasons why carbon is often referred to as the backbone of life, as it can bond with a multitude of different elements.
1.3 Common Misconceptions about Carbon Electrons
One common misconception is that all electrons in an atom are involved in bonding. While it is accurate that the outermost electrons, or valence electrons, are primarily responsible for interactions with other atoms, the inner electrons also play a role in stabilizing the atom and determining its size. Furthermore, many fail to recognize that the behaviors of these electrons are governed by quantum mechanics, influencing their probability distributions around the nucleus.
2. What is the Electron of Carbon?
2.1 Atomic Number and Electron Count
The atomic number of carbon is 6, indicating that a neutral carbon atom has six electrons. This equality of protons and electrons is crucial for maintaining the neutrality of the atom. In summary, when discussing what is the electron of carbon, we can confidently state that a carbon atom has six electrons.
2.2 Electron Orbitals Explained
Electron orbitals are regions around the nucleus where electrons are likely to be found. For carbon, the electron configuration is written as 1s² 2s² 2p². The ‘s’ orbitals are spherical and can hold a maximum of two electrons, while ‘p’ orbitals are dumbbell-shaped and can hold up to six electrons. Therefore, carbon has two electrons in the first energy level (1s) and four electrons in the second energy level (2s and 2p), allowing it to form stable bonds with other elements.
2.3 Valence Electrons and Their Significance
Carbon’s four valence electrons (the electrons in the outermost shell) are pivotal for its ability to form diverse molecules. This unique characteristic supports the formation of chains and rings in organic chemistry, facilitating the creation of a vast array of compounds. The tetravalency of carbon not only explains the complexity of organic matter but also its stability, as carbon can form single, double, or even triple bonds with other atoms.
3. The Electron Configuration of Carbon
3.1 Step-by-Step Guide to Writing Electron Configurations
To write the electron configuration of carbon, one must follow these systematic steps:
- Identify the element’s atomic number (for carbon, it is 6).
- Begin filling the lowest energy orbitals first, adhering to the Pauli Exclusion Principle and Hund’s Rule.
- The electrons will fill the 1s orbital first, accommodating 2 electrons, then 2 electrons in the 2s orbital, and finally, the remaining 2 electrons will occupy the 2p orbital.
- Thus, the completed electron configuration for carbon is 1s² 2s² 2p².
3.2 Understanding 1s, 2s, and 2p Orbitals
The ‘s’ orbitals are spherical, which means they are evenly distributed around the nucleus. The 2s orbital is larger than the 1s due to its higher energy level but only accommodates two electrons. On the other hand, the ‘p’ orbitals are more complex; they can hold three pairs of electrons (total of six) and come in sets of three oriented at right angles. In carbon, two electrons occupy the 2s orbital and two electrons occupy one of the three 2p orbitals, thereby facilitating its bonding behavior.
3.3 Electron Configuration Patterns in Non-Metals
In non-metals like carbon, patterns in electron configuration often reflect higher electronegativities and the tendency to gain or share electrons during chemical bonding. Non-metals possess higher ionization energies, making it harder to remove the outermost electrons. Thus, these patterns not only illustrate electron arrangements but also the chemical properties and reactions characteristic of non-metals.
4. Role of Carbon Electrons in Chemistry
4.1 How Electrons Influence Carbon Bonds
The ability of carbon atoms to form bonds with other atoms is primarily dictated by the arrangement of their electrons. Covalent bonds occur when carbon shares electrons with other elements. For example, when carbon binds with hydrogen, it can form methane (CH₄), where each hydrogen atom shares one electron with carbon’s four valence electrons. This sharing allows for stable molecular configurations, demonstrating how electrons are foundational to chemical bonding.
4.2 The Role of Electrons in Organic Chemistry
In organic chemistry, the arrangement and behavior of carbon’s electrons are critical in defining compound properties. Functions such as acidity and basicity are often determined by how carbon-based molecules can donate or accept electrons. Moreover, the formation of functional groups—specific combinations of carbon and other elements—further illustrates how electron dynamics shape organic compounds and their reactivity.
4.3 Electrons in Biological Processes and Molecules
Carbon’s ability to form various types of bonds underpins the structure of many biological macromolecules, including carbohydrates, lipids, proteins, and nucleic acids. The structure-function relationship in biochemistry heavily relies on how electrons contribute to molecular shape and stability. For instance, the electron movements in photosynthesis allow plants to convert sunlight into chemical energy, highlighting the central role of carbon’s electrons in life’s biochemical processes.
5. Common Questions and FAQs about Carbon Electrons
5.1 How Many Electrons Can Carbon Have?
While a neutral carbon atom contains six electrons, the number can vary in ionic compounds. For example, a carbon atom may lose or gain electrons to form cations or anions, respectively. However, in its most common form, organic chemistry primarily deals with carbon in its neutral state with six electrons.
5.2 Frequently Asked Questions about Electron Configuration
Many students and enthusiasts often grapple with questions surrounding electron configurations. Common queries include how to read configurations like 1s² 2s² 2p², and how changes in these configurations affect chemical behavior. A good grasp of quantum mechanics principles, such as the importance of energy levels and electron orbitals, can clarify many misunderstandings.
5.3 Resources for Further Learning on Carbon and Electrons
For those looking to deepen their understanding of carbon and its electrons, numerous resources are available, including textbooks on chemistry, articles, and online educational platforms. Exploring multimedia resources, such as videos on electron configurations and interactive simulations can also enhance comprehension.