Introduction to Orbital Notation of Carbon
The study of atomic structure and electron configurations is crucial in understanding chemical bonding and reactivity. One of the fundamental concepts in this sphere is the orbital notation of carbon, which succinctly describes how electrons are arranged around the atom’s nucleus. This article delves into orbital notation, its significance in the realm of chemistry, and its applications, particularly concerning carbon – an element critical to life and organic chemistry.
What is Orbital Notation?
Orbital notation is a method of expressing the electron configuration of an atom. It uses a diagrammatic or written format to show the distribution of electrons among various orbitals within different energy levels. Each atomic orbital can hold a maximum of two electrons, described using arrows that denote electron spins. This notation facilitates the understanding of electron arrangement in atoms, thus assisting in predicting chemical behavior.
Importance of Electron Configuration
Electron configurations provide essential insights into how atoms interact chemically. The arrangement of electrons influences the chemical properties of an element, including its ionization energy, electronegativity, and reactivity. For example, understanding why carbon can form four covalent bonds hinges on its electron configuration, which reveals two unpaired electrons available for bonding in its outer shell. Thus, knowing the orbital notation is foundational for any chemist.
Overview of Carbon’s Structure
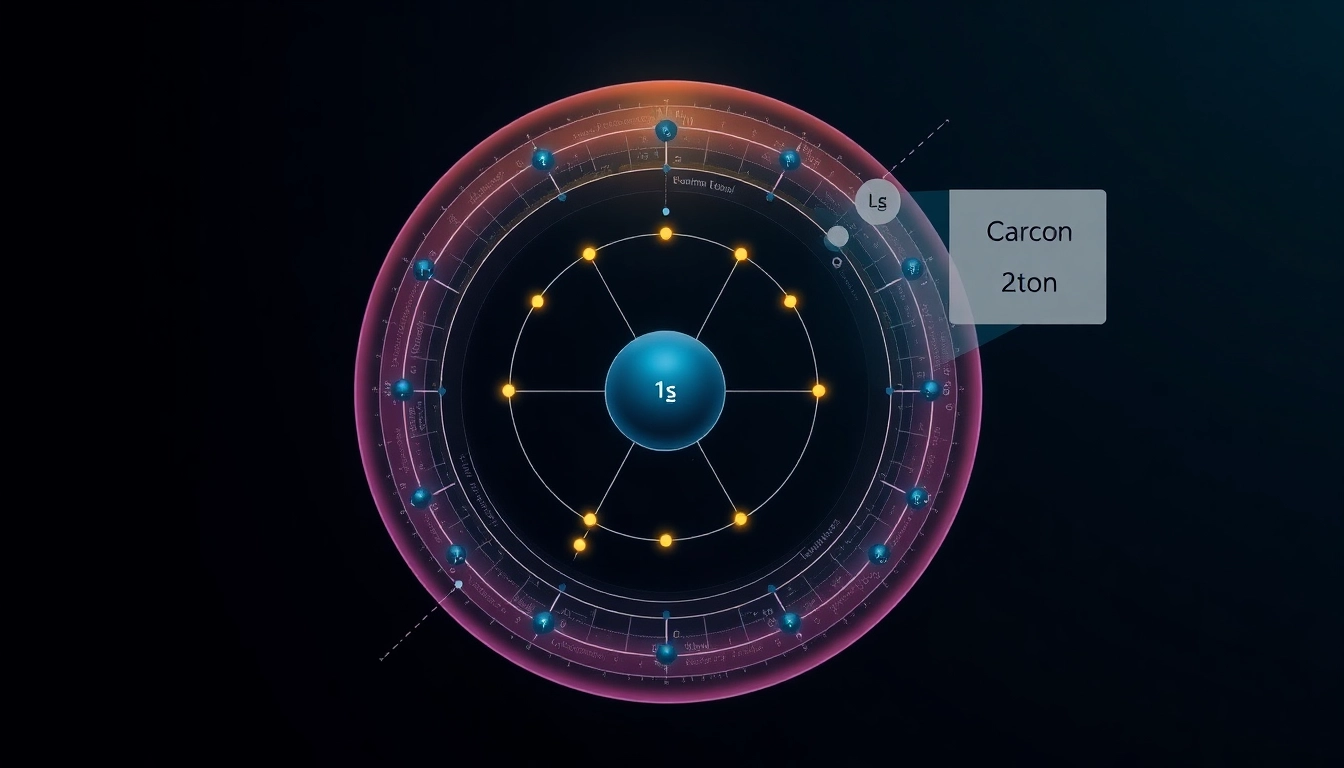
Carbon, with the atomic number 6, is the fourth most abundant element in the universe by mass. It has an electron configuration of 1s2 2s2 2p2, indicating that it possesses two electrons in the 1s orbital, two in the 2s orbital, and two in the 2p orbitals. The configuration means that carbon has a total of six electrons, with the first two (1s) closely bound to the nucleus, the next two (2s) also relatively stable, and the last two (2p) being in a higher energy state, which makes them more available for bonding. This characteristic of carbon contributes to its unique ability to form diverse compounds.
Basic Electron Configuration for Carbon
Understanding 1s, 2s, and 2p Orbitals
To fully grasp the orbital notation for carbon, one must first understand the specific characteristics of its orbitals. The terms 1s, 2s, and 2p refer to the shape and energy levels of the atomic orbitals.
- 1s Orbital: This is the closest orbital to the nucleus and is spherical in shape. It can hold a maximum of two electrons, which are tightly held due to their proximity to the positive charge of the nucleus.
- 2s Orbital: Also spherical, the 2s orbital is slightly higher in energy than the 1s orbital. It can also hold a maximum of two electrons.
- 2p Orbitals: The 2p orbitals include three distinct orientations (2px, 2py, and 2pz), each shaped like a dumbbell. Altogether, the 2p orbitals can accommodate up to six electrons.
Orbital Filling Order
Electrons fill atomic orbitals in an order based on increasing energy levels, a process guided by the aufbau principle. According to this principle, electrons occupy lower-energy orbitals first before moving to higher ones. Therefore, carbon fills its orbitals following this order:
- 1s
- 2s
- 2p
The electron configuration that arises from this filling order can be represented as 1s2 2s2 2p2, illustrating that carbon has a total of six electrons: two in the 1s orbital, two in the 2s orbital, and two within the 2p orbitals.
Notational Practices
In chemistry, standard notation practices are critical for effective communication. Orbital notation can be represented through different methods:
- Electron Configuration Notation: Expressed as 1s2 2s2 2p2, as previously mentioned, this shorthand notation specifies the number of electrons in each orbital.
- Orbital Diagrams: In diagrams, orbitals are represented as boxes or lines, with arrows indicating the electrons. Each orbital can hold a maximum of two electrons, represented with arrows pointing in opposite directions to denote their spins. This visual representation is particularly useful for understanding how electrons occupy the orbitals following Hund’s rule.
The notational system adopts standards such as IUPAC’s rules which help avoid variations that could lead to confusion among scientists.
Detailed Orbital Notation of Carbon
Full Configuration for Carbon (C)
The full orbital notation for carbon is as follows:
1s² 2s² 2p²
This denotes that carbon has two electrons in the 1s orbital, two in the 2s orbital, and two in the 2p orbitals. The notation quickly conveys all relevant information regarding its electron configuration, which is essential for understanding its chemical properties. This structure sets the foundation for carbon’s ability to engage in four covalent bonds, a characteristic vital for organic compounds.
Visual Representation: Orbital Diagram
Visualizing carbon’s electron configuration through an orbital diagram aids in grasping how the electrons are distributed across the orbitals. The orbital diagram for carbon would look like this:
1s: ↑↓
2s: ↑↓
2p: ↑ ↑
In this diagram, each box represents an orbital, and the arrows represent the electrons. An empty box indicates that the orbital has no electrons, while a filled box indicates that it is fully occupied. The two arrows in the 2p indicate the presence of the two unpaired electrons, which are crucial for the formation of bonds.
Common Misconceptions
Several misconceptions about the orbital notation of carbon may arise, such as:
- All Orbitals Are Filled: A common misunderstanding is that all orbitals are filled in an atom. In the case of carbon, while the 1s and 2s orbitals are filled, the 2p orbitals are not fully filled.
- Electrons Are Randomly Distributed: Many might assume that electrons are randomly spread throughout the orbitals. However, they occupy energy levels based on specific rules (the Aufbau principle and Hund’s rule), ensuring that the most stable configurations are reached.
- Orbitals Are Fixed orbs of Space: It is crucial to understand that orbitals are not fixed paths or “orbits” like planets around the sun; they are regions of probability where finding an electron is most likely.
Addressing these misconceptions helps students develop a more accurate understanding of atomic structure and chemical behavior.
Applications of Orbital Notation in Chemistry
Electron Configurations in Bonding
The structure provided by orbital notation serves as a vital tool in predicting how atoms will bond. For carbon, having four unpaired electrons allows for the formation of four covalent bonds, leading to the vast number of organic molecules. This ability is the basis for the complexity of biological molecules, as it allows for the creation of long chains and complex three-dimensional structures essential for life.
Role in Chemical Reactions
Orbital notation also plays a crucial role in understanding chemical reactions. The reactivity of atoms is influenced by their electron configurations. Elements that have unpaired electrons, like carbon, are more reactive as they seek to achieve a stable electron configuration. This knowledge aids chemists in predicting the outcomes of reactions, understanding reaction mechanisms, and designing new compounds.
Predicting Element Behavior
Beyond individual reactions, electron configurations help in classifying elements into different groups based on their valence electrons. The periodic table is structured such that elements in the same group typically exhibit similar chemical behaviors. For instance, carbon’s tetravalency showcases its distinct properties, differentiating it from elements in neighboring groups. This predictive ability is fundamental in various fields of chemistry, including material science and biochemistry.
Conclusion and Further Reading
Summary of Key Points
Understanding the orbital notation for carbon is essential for grasping fundamental concepts in chemistry, especially those related to bonding and reactivity. The notation provides insights into the arrangement of electrons, thereby informing predictions about an element’s chemical behavior.
Recommended Resources for Learning
For those seeking deeper knowledge in this area, several resources can enhance understanding:
- Chem Chemistry LibreTexts – Offers comprehensive chemistry resources.
- Khan Academy – Provides video tutorials about electron configurations and bonding.
- YouTube Tutorials – Search for carbon’s orbital notation and configurations for detailed visual guides.
Encouragement to Explore Orbital Theory
Lastly, delving deeper into the world of electrons, orbitals, and their notations opens a path to master chemistry. Understanding these principles not only strengthens foundational knowledge but also empowers one to explore advanced topics in chemical bonding and molecular interactions. The exploration of chemistry is vast and rewarding, from organic syntheses to biochemical pathways in living organisms.