An Overview of Electron Configuration
What is Electron Configuration?
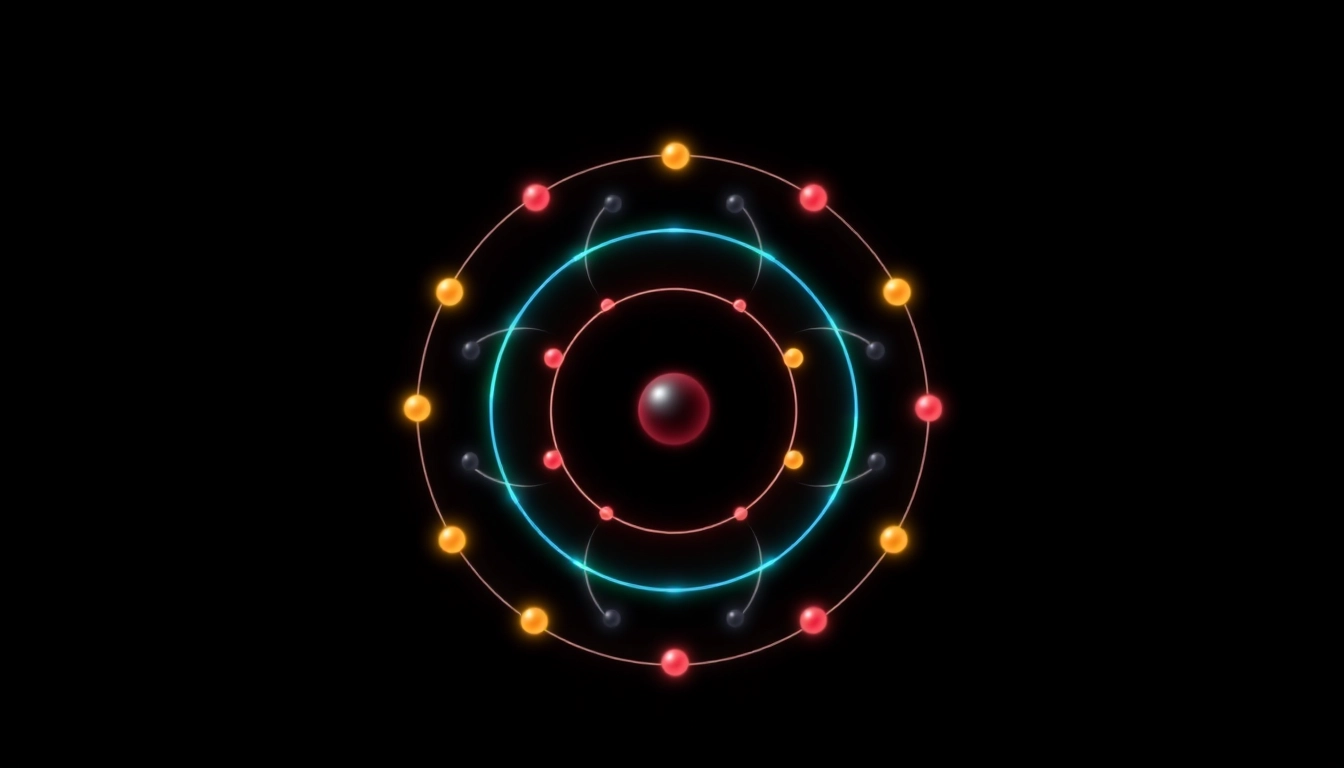
Electron configuration refers to the arrangement of electrons in an atom. It describes how electrons are distributed among the different atomic orbitals and energy levels. The configuration is expressed in terms of sublevels such as s, p, d, and f, and the number of electrons in each sublevel. Understanding electron configuration is crucial for predicting how an atom will react chemically, as the properties and behavior of elements are largely influenced by their electron arrangements. Thus, for carbon, which has the atomic number 6, its full full electron configuration of carbon reveals much about its chemical characteristics.
The Importance of Understanding Electron Configuration
Grasping electron configuration is fundamental in chemistry and physics. It serves as the bedrock for understanding the periodic table, bonding behavior, and the principles governing molecular interactions. Moreover, electron configuration helps explain why elements within the same group exhibit similar chemical properties. For instance, the reactivity and types of bonds an element can form are often rooted in the distribution of its electrons. As such, a thorough knowledge of electron configuration is indispensable for students, educators, and professionals in the scientific community.
Common Terms in Electron Configuration
- Atomic Number: The number of protons in an atom, which determines the element’s identity.
- Orbitals: Regions in an atom where electrons are likely to be found. Common types include s, p, d, and f orbitals.
- Energy Levels: These denote the distance of electrons from the nucleus, with higher levels corresponding to higher energy states.
- Hund’s Rule: A principle stating that electrons will occupy degenerate orbitals singly before pairing up.
- Aufbau Principle: A rule that dictates the order in which electrons occupy orbitals, filling lower-energy orbitals first.
The Full Electron Configuration of Carbon
Ground State Configuration of Carbon
Carbon, with an atomic number of 6, has a total of six electrons. In its ground state, these electrons are arranged according to the Aufbau principle, starting from the lowest energy levels. The full electron configuration of carbon is written as 1s² 2s² 2p². This notation represents the number of electrons in each sublevel: two electrons occupy the 1s orbital, two electrons fill the 2s orbital, and the remaining two electrons occupy the 2p orbital. Understanding this arrangement is fundamental in predicting the chemical behavior of carbon, a key element in organic chemistry.
Orbital Filling Order and Energy Levels
The order in which electrons fill the orbitals is vital in determining the final electron configuration. According to the Aufbau principle, the filling order progresses from the lowest to higher energy levels. The sequence typically follows the pattern: 1s, 2s, 2p, 3s, 3p, and so on. For carbon, the electrons occupy the orbitals in this order until the configuration of 1s² 2s² 2p² is achieved. This structured filling helps illustrate why carbon has the unique properties that make it essential for life as we know it.
Summary of Carbon’s Electron Configuration: 1s² 2s² 2p²
To summarize, carbon’s electron configuration of 1s² 2s² 2p² reveals how its electrons are structured within its atomic orbitals. This configuration signifies that carbon is tetravalent, meaning it can form four bonds with other elements. The presence of two electrons in the p-orbital plays a crucial role in carbon’s versatility in forming various organic compounds. This unique electron arrangement allows carbon to bond with a wide variety of elements, giving rise to the immense diversity of life on Earth.
How to Write Electron Configurations: Step-by-Step
Using the Aufbau Principle
The Aufbau principle is foundational for writing electron configurations. To apply this principle, one must begin with the lowest energy orbital and progressively fill higher energy orbitals as electrons are added. Here’s a step-by-step guide to writing an electron configuration:
- Identify the atomic number of the element to determine the total number of electrons. For carbon, this is 6.
- Fill the 1s orbital first, which can hold up to two electrons: 1s².
- Next, fill the 2s orbital with two electrons: 2s².
- Then, distribute the remaining two electrons in the 2p orbital: 2p².
Following these steps will lead to the electron configuration of carbon being represented accurately as 1s² 2s² 2p².
Applying Hund’s Rule
Recognizing how to apply Hund’s rule is essential when dealing with orbitals that can hold more than one electron, such as the 2p orbitals in carbon’s case. Hund’s rule states that electrons will fill degenerate orbitals singly before a second electron occupies the same orbital. In the case of carbon, once two electrons fill the 1s and 2s orbitals, the 2p orbitals are filled according to Hund’s rule. This means that the two electrons in the 2p level will occupy separate p orbitals until all are half-filled; thus, carbon’s configuration emphasizes its ability to form covalent bonds effectively.
Example: Writing the Full Electron Configuration for Carbon
Let’s work through the steps to write the full electron configuration for carbon practically:
- Starting with an atomic number of 6, we identify that carbon has 6 electrons.
- Applying the Aufbau principle, we fill the 1s orbital first with 2 electrons: 1s².
- Next, we fill the 2s orbital with the next 2 electrons: 2s².
- We have now placed 4 electrons. The next 2 electrons will fill the 2p orbital, which gives us: 2p².
Consequently, the complete electron configuration for carbon is succinctly written as 1s² 2s² 2p², illustrating the arrangement of electrons around the carbon nucleus.
Common Questions About Carbon’s Electron Configuration
Why is Carbon’s Configuration Significant in Chemistry?
The electron configuration of carbon is critical because it directly influences carbon’s role as a fundamental building block in organic chemistry. The four valence electrons in carbon allow it to form strong covalent bonds with other atoms, including other carbon atoms. This unique characteristic enables the formation of long carbon chains and complex molecules, such as proteins, carbohydrates, and nucleic acids, which are essential for life. The versatility in bonding also accounts for carbon’s presence in various allotropes, such as diamond and graphite, showcasing its adaptability in different structural forms.
How Does Carbon’s Electron Configuration Affect Its Properties?
Carbon’s electron configuration results in several unique properties, mainly due to the arrangement of its valence electrons. Carbon’s ability to form four bonds leads to the creation of an extensive range of organic compounds with diverse structures and functions. Additionally, this tetravalency allows for hybridization of orbitals, enabling the formation of single, double, and triple bonds, which provide the backbone for larger biochemical structures. Its relatively high electronegativity also means it can engage in various interactions, influencing how molecules behave in chemical reactions.
Exploring Carbon’s Excited States and Variations
While carbon typically exists in its ground state configuration (1s² 2s² 2p²), it is essential to note that electrons can be excited to higher energy levels. For instance, when energy is supplied, one of the 2s electrons may transition to a higher energy level, contributing to variations in bonding and reactivity. This phenomenon is crucial in understanding reactions like combustion and photosynthesis. Additionally, carbon can form various ions, such as cations and anions, that further alter its reactivity based on the gain or loss of electrons, reflecting its need to achieve a stable electron configuration akin to that of noble gases.
Further Learning on Electron Configuration
Resources for Advanced Chemistry Studies
For students and educators seeking to deepen their understanding of electron configurations and advanced chemistry concepts, several resources are available:
- LibreTexts Chemistry – offers comprehensive modules on electron configurations and periodic trends.
- Khan Academy – provides video tutorials and practice exercises.
- Chemguide – an excellent resource for detailed explanations and chemical principles.
Online Tools and Simulations
Several online tools and simulations can aid in visualizing and understanding electron configurations effectively:
- PhET Interactive Simulations: Offers virtual simulations for exploring atomic structure and electron configuration.
- Wolfram Alpha: A computational engine that can generate various chemical properties and configurations.
- Royal Society of Chemistry Interactive Periodic Table: A resource that provides key information and illustrations for each element.
Networking with Chemistry Communities
Engaging with fellow chemistry enthusiasts or professionals can enhance understanding and provide support. Online forums and social media networks such as Reddit, Facebook groups, and LinkedIn groups dedicated to chemistry can connect you with experts and peers who share your interests. Furthermore, participating in local or regional chemical societies can help build a solid foundation and offer networking opportunities that may lead to further learning and collaboration.