1. Introduction to Carbon and Its Importance
Carbon is a fundamental building block of life, forming the backbone of organic molecules. It is the sixth element in the periodic table, denoted by the symbol ‘C’. With six protons and six electrons, carbon possesses unique properties that make it versatile in its chemical behavior. Understanding carbon’s electronic structure is crucial for comprehending its role in chemistry, biology, and materials science. This article delves into the nuances of carbon’s electron configuration and its implications across various fields.
1.1 What is the Electronic Structure of Carbon?
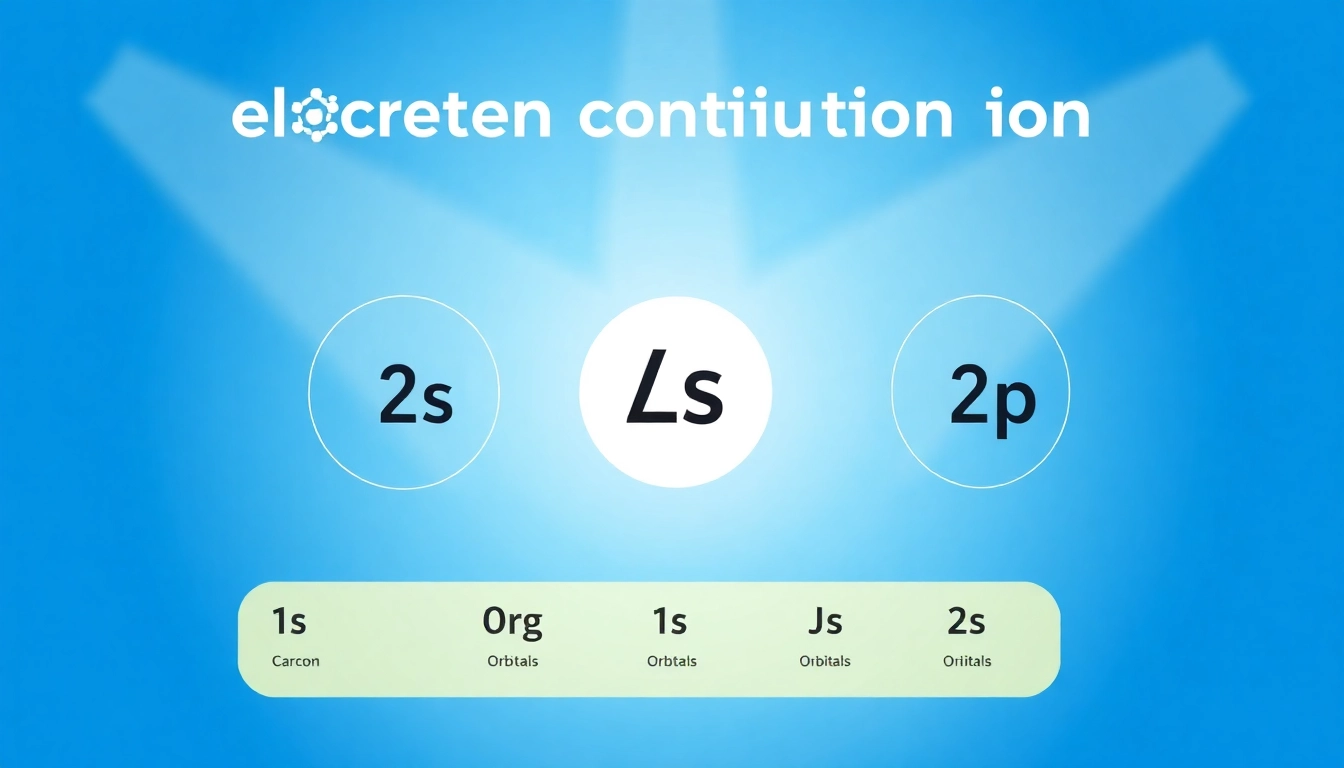
The electronic structure of carbon refers to the arrangement of its electrons within atomic orbitals. Carbon’s atomic number is six, which signifies that it has six electrons. These electrons are distributed in two shells: the first shell can hold up to two electrons, while the second shell can accommodate eight. The configurations of the electrons in carbon are represented as follows:
1s² 2s² 2p²
In this notation, ‘1s’ indicates the first energy level (K shell) and the ‘s’ subshell having 2 electrons. ‘2s’ and ‘2p’ represent the second energy level (L shell) with 2 electrons in the ‘s’ subshell and 2 electrons in the ‘p’ orbitals, respectively. This arrangement is key in understanding carbon’s ability to form covalent bonds with other elements.
1.2 Why Carbon is Essential in Chemistry
Carbon is often referred to as the ‘element of life’ because it is present in all living organisms. Its unique ability to form stable bonds with many elements, including itself, allows for the creation of complex molecules such as carbohydrates, proteins, lipids, and nucleic acids. These biomolecules are crucial for life processes, making carbon indispensable in biochemistry and molecular biology.
1.3 Overview of Electron Configurations
In chemistry, electron configurations describe the distribution of electrons within an atom’s orbitals. Understanding electron configurations is fundamental for predicting an element’s chemical properties and behavior. Elements are structured in a way that their electron configurations follow specific rules, such as the Aufbau principle, Pauli exclusion principle, and Hund’s rule. This systematic approach allows chemists to determine how elements will interact in chemical reactions based on their electron arrangements.
2. Detailed Electron Configuration of Carbon
2.1 Ground State Configuration Explained
The ground state configuration of an atom is its lowest energy arrangement of electrons. For carbon, this configuration is represented as 1s² 2s² 2p², indicating that all electrons are in their lowest possible energy levels. This ground state is significant because it defines how carbon will behave in chemical interactions. The configuration indicates that carbon has four valence electrons, which are responsible for its chemical bonding characteristics.
2.2 Writing the Electron Configuration for Carbon
To write the electron configuration for carbon, one must start from hydrogen (the first element) and proceed sequentially through to carbon, filling the orbitals according to their energy levels. Following the principles of electron filling:
- The 1s orbital is filled first with 2 electrons.
- The 2s orbital is next and also holds 2 electrons.
- The 2p orbitals (which include 2px, 2py, and 2pz) are filled with the remaining 2 electrons, each occupying one of the p orbitals before pairing up according to Hund’s rule.
This systematic filling leads to the conclusion that carbon has a ground state electron configuration of 1s² 2s² 2p².
2.3 Common Misconceptions About Carbon’s Structure
Despite its widespread study, several misconceptions about carbon’s electronic structure persist. Some common misconceptions include:
- Carbon does not bond with itself: This is incorrect; carbon can form stable covalent bonds with other carbon atoms, resulting in a variety of molecular structures from simple hydrocarbons to complex organic compounds.
- All carbon’s electrons are in the outer shell: While the four valence electrons play a critical role in bonding, carbon also has inner shell electrons that influence its overall energy state and chemical behavior.
- The p orbitals can only hold 2 electrons: Each p orbital (2px, 2py, and 2pz) can hold up to 2 electrons, but in carbon’s case, they accommodate 2, demonstrating an essential aspect of electron pairing.
3. Carbon’s Electronic Structure and Chemical Properties
3.1 How Electron Configuration Influences Bonding
The electron configuration directly impacts how carbon atoms bond with other elements. Carbon’s four valence electrons enable it to make four covalent bonds, facilitating the formation of stable compounds, such as methane (CH₄), carbon dioxide (CO₂), and numerous organic compounds. The tetrahedral shape of methane is a direct consequence of sp³ hybridization, which rearranges electrons for optimal bonding efficiency.
3.2 Valence Electrons and Their Role in Reactivity
Valence electrons are the outermost electrons of an atom and are crucial in determining its reactivity and how it interacts with other elements. Carbon’s four valence electrons allow it to engage in various types of chemical bonding, including single, double, and triple bonds. This flexibility is fundamental to organic chemistry, where carbon can form diverse structures through various bonding arrangements.
3.3 Examples of Carbon Compounds
Carbon’s versatile bonding capabilities lead to the formation of a wide array of compounds:
- Hydrocarbons: Compounds consisting solely of carbon and hydrogen, such as ethylene (C₂H₄) and acetylene (C₂H₂), showcasing carbon’s ability to form double and triple bonds.
- Carbohydrates: Organic molecules composed of carbon, hydrogen, and oxygen, such as glucose (C₆H₁₂O₆), essential for life as energy sources.
- Proteins: Large biomolecules composed of carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur, illustrating the complexity of carbon-rich structures.
4. Carbon in Different States and Ions
4.1 Electron Configuration in Excited States
While carbon’s ground state electron configuration is 1s² 2s² 2p², carbon can also exist in excited states where electrons are promoted to higher energy levels. For example, one can find configurations such as 1s² 2s¹ 2p³, where an electron from the 2s level has moved to an empty 2p orbital. Such excited states can influence the reactivity of carbon and its ability to form different types of bonds during various chemical reactions, such as in biochemical processes.
4.2 How Carbon Ions Differ from Neutral Carbon
Neutral carbon, having six protons and six electrons, exhibits a balanced charge. However, when carbon forms ions, its electron configuration changes. For example:
- Carbon cation (C⁺): Contains only five electrons, typically resulting from the loss of an electron. Its configuration might be 1s² 2s² 2p¹, leading to increased reactivity.
- Carbon anion (C⁻): Has seven electrons, indicating an additional electron. The configuration is 1s² 2s² 2p³, making it more stable but reactive in certain chemical contexts.
4.3 Application in Organic Chemistry
Understanding carbon’s electronic structure is pivotal in organic chemistry, where carbons’ ability to form stable bonds dictates the behavior of countless molecules. Reactions involving carbon is often characterized by the types of hybridization:
- sp³ Hybridization: Carbon forms four single bonds.
- sp² Hybridization: Carbon forms two single bonds and one double bond.
- sp Hybridization: Carbon forms one single bond and one triple bond.
This knowledge allows chemists to synthesize new compounds and predict their behavior in biological and chemical processes.
5. Conclusion and Further Reading
5.1 Summary of Carbon’s Electronic Structure
Carbon is undoubtedly a unique element, central to the chemistry of life. Its electronic structure, characterized by a configuration of 1s² 2s² 2p², provides it with extraordinary versatility in chemical bonding. This ability fuels the vast diversity of organic compounds found in nature, highlighting carbon’s role as a cornerstone of biological chemistry.
5.2 Resources for Further Study
For readers interested in deepening their understanding of carbon’s electronic structure and its implications, the following resources may prove valuable:
- Electron Configuration for Carbon – TerpConnect
- Carbon Electron Configuration Diagram – Science Photo
- Understanding the Structure and Bonding of Carbon – StudyPug
5.3 FAQs on Carbon Electron Configurations
Q: What is the electron configuration of carbon?
A: The electron configuration of carbon is 1s² 2s² 2p².
Q: How many electrons can carbon share in bonds?
A: Carbon can share up to four electrons through covalent bonding, allowing it to make a total of four bonds with other atoms.
Q: Why is carbon important in organic chemistry?
A: Carbon’s versatile bonding capabilities enable the formation of a wide range of organic molecules, making it essential for biological processes and the foundation of organic chemistry.