1. Introduction to Electron Configuration
The electronic configuration of carbon is fundamental to understanding the behavior of this essential element in both chemical reactions and biological processes. Electron configurations describe the distribution of electrons in an atom’s orbitals, providing insights into its structure, reactivity, and bonding capabilities. With carbon being a cornerstone of organic chemistry and life itself, an in-depth comprehension of its electronic configuration is crucial for students, researchers, and enthusiasts alike.
1.1 What is Electron Configuration?
Electron configuration refers to the arrangement of electrons in the atomic orbitals of an atom. Each orbital, which is a region in space where electrons are likely to be found, can hold a maximum number of electrons: the s orbitals can hold 2 electrons, p orbitals can hold 6, d orbitals can hold 10, and f orbitals can hold 14. The configuration is typically expressed in the form of energy levels and subshells, such as 1s² 2s² 2p¹, indicating the distribution of electrons in these orbitals.
1.2 Importance of the Electronic Configuration of Carbon
Understanding the electronic configuration of carbon is vital due to the element’s unique ability to form stable bonds with a diverse array of other elements. This bonding versatility is largely attributed to carbon’s four valence electrons, which allow it to create four covalent bonds, forming complex molecules that are foundational to life. The electronic configuration delineates how these bonds can form and the type of molecules that can result from carbon’s interactions.
1.3 Overview of Atomic Structure
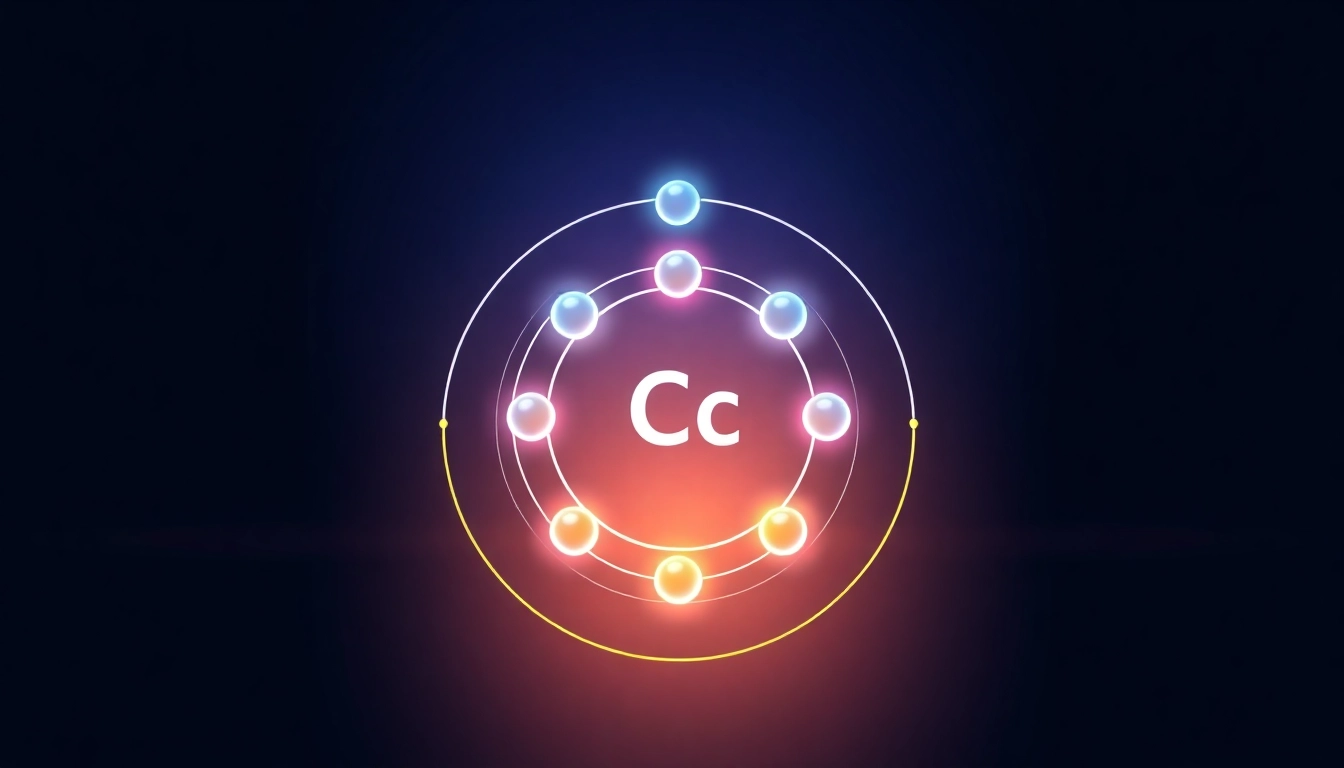
The structure of an atom consists of a nucleus, made up of protons and neutrons, surrounded by electrons that reside in various orbitals. The atomic number, which is the number of protons in the nucleus, determines the element’s identity. For carbon, the atomic number is 6, meaning it has 6 protons and, typically, 6 electrons. This structure gives rise to the unique characteristics of carbon, allowing it to participate in a variety of chemical reactions.
2. Carbon Basics: Atomic Number and Structure
2.1 The Significance of Atomic Number 6
The atomic number of carbon is 6, which signifies that a neutral carbon atom contains 6 protons. This is significant because it defines not only the identity of the atom but also its position on the periodic table, between boron (atomic number 5) and nitrogen (atomic number 7). The arrangement of electrons corresponding to this atomic number informs us about the chemical properties of carbon, influencing its reactivity and the types of bonds it can form.
2.2 Overview of Carbon’s Electron Shells
Carbon’s electrons are distributed in two energy levels or shells: the first shell (1s) contains 2 electrons, while the second shell (2s and 2p) contains 4 electrons (2 in the 2s orbital and 2 in the 2p orbitals). This configuration is expressed as 1s² 2s² 2p². The filled first shell provides a stable base, while the partially filled second shell facilitates chemical bonding. The specific arrangement of electrons in these shells helps determine carbon’s unique properties and its ability to form four covalent bonds.
2.3 Common Characteristics of Carbon
Carbon stands out among elements for several reasons: it has the ability to readily form stable, covalent bonds with itself and other elements, leading to extensive networks of molecules. Known as the “building block of life,” carbon’s versatility allows it to create the backbone of biological macromolecules such as carbohydrates, proteins, nucleic acids, and lipids. Additionally, its relatively small atomic size, combined with its electronegativity, contributes to its bonding capabilities.
3. Writing the Electronic Configuration of Carbon
3.1 Step-by-Step Guide to Electron Configuration
To write the electron configuration for carbon, follow these steps:
- Identify the atomic number of carbon, which is 6.
- Begin filling the 1s orbital with electrons. Since it holds a maximum of 2 electrons, we write 1s².
- Next, move to the 2s orbital and fill it similarly with 2 electrons, yielding 2s².
- Now, for the 2p orbital, we need to place the remaining 2 electrons into this orbital: resulting in 2p².
Thus, the complete electron configuration for carbon is 1s² 2s² 2p².
3.2 Orbital Diagrams and Notation Explained
Orbital diagrams visually represent the arrangement of electrons in an atom. Each box represents an orbital, and arrows depict electrons, indicating their spin direction. For carbon, an orbital diagram would illustrate the 1s energy level filled with two arrows pointing in opposite directions, the 2s also filled with two arrows, and the 2p orbitals holding a total of two electrons. Particularly useful for visual learners, these diagrams complement the traditional electron configuration notation by providing an immediate visual cue of electron arrangement and pairing.
3.3 Common Misunderstandings in Electron Configuration
One common misunderstanding when dealing with electron configurations is the significance of Hund’s rule. According to this rule, when electrons occupy orbitals of the same energy (like the three 2p orbitals), they will first fill each orbital singly before pairing up. Thus, while carbon’s typical ground state configuration is indeed 1s² 2s² 2p², a common representation using arrows would show two of the p orbitals each with 1 electron before the second electron fills one of them. Understanding these subtleties is key to mastering the topic, especially for chemistry students.
4. Practical Applications of Electron Configuration
4.1 Electron Configuration in Chemical Bonding
Electron configuration plays a pivotal role in defining how atoms bond with one another. For carbon, the four valence electrons allow various bonding configurations, including single, double, and triple bonds. This versatility is particularly evident in organic chemistry, where carbon can form long chains and complex structures, which are crucial in organic molecules. This section of carbon’s configuration allows for the formation of hybridized orbitals like sp³, sp², and sp, facilitating different geometric structures.
4.2 The Role of Carbon in Organic Chemistry
In organic chemistry, carbon’s unique properties, derived from its electronic configuration, enable it to bond with a myriad of other atoms to create complex molecules. Carbon can form stable covalent bonds with other carbon atoms, leading to chains and rings that serve as the primary scaffolding for organic compounds. Additionally, carbon’s ability to form bonds with elements such as hydrogen, oxygen, nitrogen, and sulfur, creates a vast array of functional groups, contributing to the diversity of chemical reactions in biological systems.
4.3 Examples of Carbon Compounds and Their Configurations
Some common compounds that illustrate the diverse applications of carbon’s electronic configuration include:
- Carbon Dioxide (CO₂): This molecule showcases double bonding as oxygen atoms attach to carbon through double bonds, emphasizing the bonding flexibility of carbon.
- Glucose (C₆H₁₂O₆): The structure of glucose highlights the extensive S and P bonding capabilities of carbon, contributing to its intricate ring structure.
- Octane (C₈H₁₈): This hydrocarbon displays how carbon can create extensive chains and branches, fundamental to fuels and energy sources.
Each example demonstrates how carbon’s electron configuration dictates the molecular structure and chemical properties of compounds, playing a critical role in everyday chemistry.
5. Frequently Asked Questions about Carbon’s Electron Configuration
5.1 Common Questions and Concerns
Many students commonly inquire about why carbon’s electron configuration is written with the 2p electrons occupying lower energy instead of pairing in a single 2p orbital. This question is rooted in the preference of atoms to minimize energy in their electron interactions, as explained by Hund’s rule and the principles of valence shell electron repulsion (VSEPR) theory.
5.2 New Discoveries in Carbon Research
Recent advancements in carbon research, especially in the fields of nanotechnology and materials science, have unveiled exciting applications of carbon-based compounds. Innovations such as graphene and carbon nanomaterials have emerged thanks to the versatility denoted by carbon’s electron configurations, leading to groundbreaking improvements in electronics and structural materials.
5.3 Resources for Further Learning about Carbon
For readers looking to explore the topic of carbon’s electronic configuration and its wider implications, several resources are available:
- Electron Configuration for Carbon (TerpConnect)
- 2.2: Electron Configurations – Chemistry LibreTexts
- Write the Ground State Electron Configuration for a Neutral Carbon – Socratic