1. Introduction to Electron Configuration
Electron configuration is a fundamental concept in chemistry that describes the distribution of electrons in an atom’s orbitals. Understanding electron configurations is critical for studying the chemical properties of elements, as it directly influences how they interact and bond with other atoms. In this article, we will dive deep into the electron configuration of carbon, which serves as a foundational building block in organic chemistry and materials science.
1.1 What is Electron Configuration?
Electron configuration refers to the arrangement of electrons in an atom’s orbitals, encompassing principal energy levels, subshells, and individual orbitals. This is denoted using a notation system that indicates the number of electrons in each energy level. Electrons occupy orbitals in a manner that minimizes energy and adheres to several principles, namely the Aufbau principle, Pauli exclusion principle, and Hund’s rule.
1.2 The Role of Electrons in Atoms
Electrons are negatively charged subatomic particles that orbit the nucleus of an atom, which is composed of protons and neutrons. The behavior and interactions of these electrons determine an atom’s chemical properties, including its reactivity, the types of bonds it can form, and the structures it can achieve. The outermost electrons, known as valence electrons, play a particularly pivotal role in chemical bonding.
1.3 Significance of Carbon in Chemistry
Carbon is the sixth element on the periodic table and has unique properties that allow it to form a vast array of compounds, including organic molecules essential for life. Its ability to form four covalent bonds with other atoms makes it incredibly versatile in creating complex structures such as proteins, nucleic acids, lipids, and carbohydrates. Understanding carbon’s electron configuration is crucial for grasping these essential chemical properties.
2. The Basics of Carbon’s Electron Configuration
2.1 Atomic Structure of Carbon
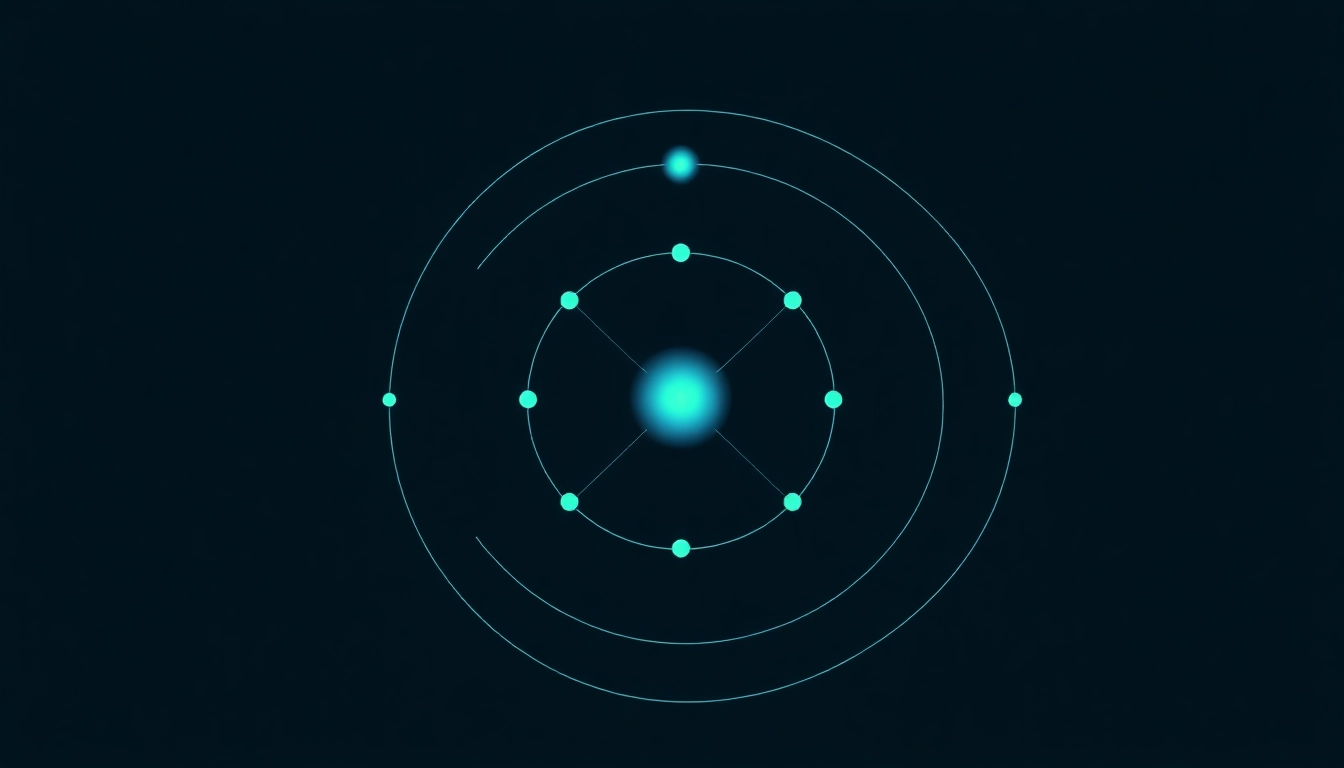
Carbon has an atomic number of 6, meaning it possesses 6 protons in its nucleus. In a neutral carbon atom, the number of electrons equals the number of protons. Therefore, a neutral carbon atom also has 6 electrons. These electrons are arranged in various energy levels, contributing to carbon’s unique chemical behavior.
2.2 Notation of Electron Configuration
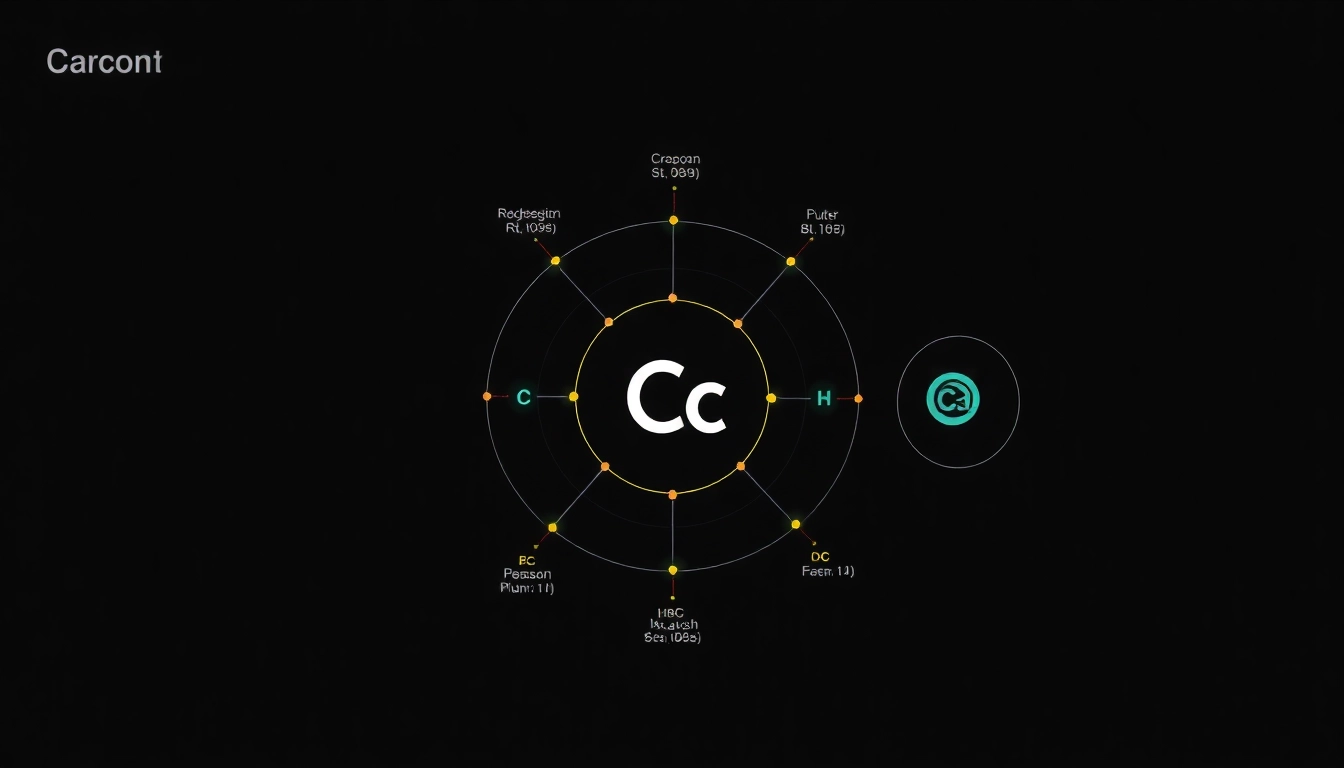
The notation for electron configuration primarily uses numbers and letters to convey the arrangement of electrons. The format includes the principal quantum number (n), the type of orbital (s, p, d, f), and the number of electrons in those orbitals. For carbon, the electron configuration is typically expressed as 1s² 2s² 2p².
2.3 The Ground State Configuration: 1s² 2s² 2p²
The ground state electron configuration of carbon, 1s² 2s² 2p², indicates that the first two electrons fill the 1s orbital, the next two electrons occupy the 2s orbital, and the remaining two electrons are in the 2p orbitals. This configuration reflects the filling order according to the Aufbau principle and provides insights into carbon’s chemical properties.
3. How to Write the Electron Configuration of Carbon
3.1 Step-by-Step Process
To write the electron configuration for carbon, follow these steps:
- Identify the atomic number: The atomic number of carbon is 6.
- Fill the orbitals starting from the lowest energy level (1s) to the higher energy levels (2s, 2p) according to the Aufbau principle.
- Distribute the electrons: Place 2 electrons in the 1s orbital, 2 in the 2s orbital, and the remaining 2 in the 2p orbitals.
- Express the configuration in standard notation: 1s² 2s² 2p².
3.2 Using the Periodic Table
The periodic table is a valuable tool for determining electron configurations. As you move from left to right across a period, electrons fill up available orbitals in a systematic manner. Carbon can be found in group 14 of the periodic table, indicating that it has four valence electrons, thus affirming its configuration.
3.3 Abbreviated vs. Full Configuration
Abbreviated or condensed electron configurations use the nearest noble gas to simplify the notation. For carbon, you can represent the electron configuration as [He] 2s² 2p², where [He] signifies the electron configuration of helium (which is 1s²). This notation maintains clarity while reducing complexity in large, multi-electron atoms.
4. Common Misconceptions About Carbon’s Electron Configuration
4.1 Variations in Notation
Students often encounter variations in the notation of electron configurations, which can lead to confusion. Some may present carbon’s configuration as 1s² 2s² 2p¹ 2p¹, emphasizing the specific filling of p orbitals. It’s crucial to remember that regardless of presentational differences, the total number of electrons remains constant—6 in this case.
4.2 Importance of Hybridization
Hybridization is a phenomenon that occurs when atomic orbitals mix to form new hybrid orbitals that can participate in bonding. Carbon often undergoes sp³, sp², or sp hybridization, depending on its bonding context. Understanding hybridization is essential when interpreting molecular geometry and reactivity.
4.3 Misinterpretations in Educational Materials
Educational resources sometimes simplify complex concepts, leading to misinterpretations. It’s essential to critically evaluate these materials while considering the underlying principles of electron configurations, hybridization, and bond formation. This awareness helps learners develop a more nuanced understanding of chemical behavior.
5. Applications of Carbon’s Electron Configuration
5.1 Role in Organic Chemistry
The electron configuration of carbon is pivotal in organic chemistry. The tetravalent nature of carbon, due to its 4 valence electrons, allows it to form stable covalent bonds with various elements, including hydrogen, oxygen, and nitrogen. This versatility is the foundation of all organic molecules, from simple hydrocarbons to complex biomolecules.
5.2 Implications for Molecular Structure
Understanding carbon’s electron configuration aids chemists in predicting molecular shapes and structures based on VSEPR (Valence Shell Electron Pair Repulsion) theory. The distribution of electrons around carbon dictates angles and geometry, providing insights into molecular dynamics and reactions.
5.3 How it Supports Advanced Chemical Theories
Advanced theories in chemistry, such as molecular orbital theory and crystallography, rely heavily on the principles of electron configuration. They allow for a deeper understanding of chemical bonding, resonance structures, and material properties, such as electrical conductivity and reactivity, which are vital in fields ranging from materials science to pharmacology.