1. Introduction to Electron Configuration
Understanding electron configurations is vital for students and professionals alike in the fields of chemistry, physics, and materials science. Electron configuration denotes the distribution of electrons in an atom’s atomic orbitals, which ultimately dictates how atoms interact with one another during chemical reactions. Among the many elements, carbon holds a special place due to its central role in organic chemistry and biological systems. In this article, we will explore the electron configuration of carbon to unveil not only what it is but also why it matters.
If you’re curious about what is the electron configuration of carbon, you are in the right place to deepen your understanding.
1.1 What is Electron Configuration?
Electron configuration describes the arrangement of electrons within an atom and is denoted by a series of numbers and letters. Each number corresponds to a principal energy level, while letters refer to the types of orbitals: s, p, d, and f. For example, the electron configuration notation 1s² indicates that there are two electrons in the first electron shell’s s orbital.
1.2 Importance of Electron Configuration in Chemistry
The configuration of electrons within an atom determines its chemical properties, reactivity, and the types of bonds it can form with other atoms. This makes understanding electron configurations fundamental to various applications, including organic chemistry, molecular biology, materials science, and even nanotechnology. For carbon, its electron configuration allows it to form stable covalent bonds, making it the backbone of organic molecules.
1.3 Overview of Carbon and Its Properties
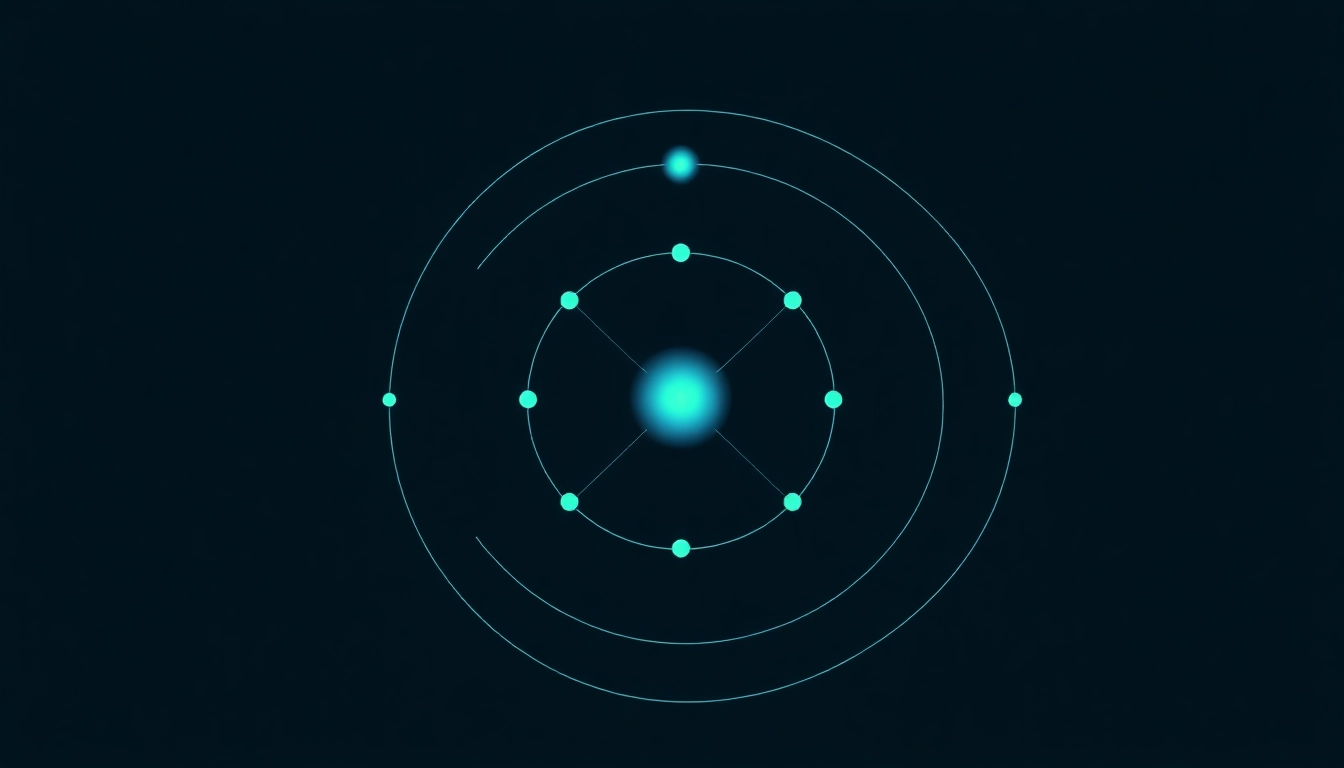
Carbon, with the atomic number 6, is a unique element that can be found in various forms known as allotropes, including graphite, diamond, and fullerenes. Its common states include gases (like carbon dioxide), solids in various structures, and a significant role in life as the basic building block of organic compounds. The electron configuration for carbon is pivotal as it lays the groundwork for its versatile bonding capabilities.
2. The Basics of Carbon’s Electron Configuration
2.1 Atomic Structure of Carbon
The atomic structure of carbon consists of six protons, six neutrons, and six electrons. The protons reside in the nucleus, while the electrons occupy different energy levels around the nucleus. The arrangement of these electrons in atomic orbitals is what we refer to when discussing an element’s electron configuration.
2.2 Filling Order of Orbitals
Orbitals are filled according to specific rules that stem from the principles of quantum mechanics. The Aufbau principle states that electrons fill the lowest energy orbitals first before occupying higher ones. For carbon, this filling order follows: 1s, 2s, and then 2p. Each of these orbitals has a defined capacity: the s-orbital can hold up to 2 electrons, while the p-orbitals can hold up to 6. The ground state electron configuration of carbon is therefore 1s² 2s² 2p².
2.3 Key Terms Related to Electron Configuration
To facilitate understanding, several key terms should be familiar:
- Orbital: The region around the nucleus where electrons are most likely to be found.
- Valence electrons: Electrons in the outermost shell, involved in bonding.
- Ground state: The lowest energy configuration of an atom’s electrons.
- Hund’s Rule: Every orbital in a given energy level is singly occupied before any orbital is doubly occupied.
3. Writing the Electron Configuration for Carbon
3.1 Step-by-Step Process for Carbon’s Configuration
To derive the electron configuration for carbon, follow these steps:
- Identify the atomic number of carbon, which is 6.
- Start filling the lowest energy level, which is 1s. Place 2 electrons in 1s (1s²).
- Move to the next energy level, 2s, and place 2 electrons (2s²).
- Continue to the 2p level and put the remaining 2 electrons in the 2p orbitals (2p²).
Thus, the complete electron configuration is 1s² 2s² 2p².
3.2 Common Notations: Full vs. Condensed Configuration
The full electron configuration is often long, as it lists all electrons in order of energy levels. Conversely, the condensed (or shorthand) notation simplifies this by using the nearest noble gas to represent core electrons. For example, the condensed version for carbon utilizes neon’s electron configuration as [He] 2s² 2p². This shorthand reduces clutter while conveying the same information.
3.3 Electron Configuration Notation Examples
Other elements present varied configurations:
- Oxygen (atomic number 8): 1s² 2s² 2p⁴ or [He] 2s² 2p⁴.
- Nitrogen (atomic number 7): 1s² 2s² 2p³ or [He] 2s² 2p³.
- Fluorine (atomic number 9): 1s² 2s² 2p⁵ or [He] 2s² 2p⁵.
These examples highlight how electron configurations can demonstrate similarities and differences between elements in the same group.
4. Visualizing Carbon’s Electron Configuration
4.1 Orbital Diagrams and Models
Visual tools can significantly enhance understanding. Orbital diagrams illustrate electrons as arrows within boxes representing orbitals. For carbon, the 1s orbital holds two electrons as paired arrows, while the 2s also holds two paired arrows. The 2p orbital diagram for carbon will show two of the three available p orbitals each holding one electron (following Hund’s rule), while the third p orbital remains empty.
4.2 Visual Aids for Understanding Configuration
Various online resources, including interactive periodic tables and molecular visualization software, can help visualize these concepts. For students or professionals seeking clarity, utilizing these tools can provide tangible representations of how electron configurations influence atomic structure.
4.3 Comparing Carbon with Other Elements
Carbon is often compared to silicon (Si) and nitrogen (N) due to their proximity on the periodic table and their electronic configurations. While carbon forms four covalent bonds thanks to its four valence electrons, silicon behaves similarly but with variations in bonding due to its larger size and the presence of d-orbitals. Meanwhile, nitrogen, with one fewer valence electron, exhibits differing chemistry, predominantly forming three bonds.
5. Applications and Implications of Carbon’s Electron Configuration
5.1 Role of Electron Configuration in Chemical Bonding
The electron configuration profoundly influences how atoms bond with each other. For carbon, the presence of four valence electrons allows the formation of stable tetravalent compounds. This adaptability leads to the abundance of organic molecules containing carbon, enabling diverse structures ranging from simple hydrocarbons to complex biomolecules.
5.2 Impacts on Organic Chemistry and Biological Systems
In organic chemistry, carbon’s configuration permits hybridization, allowing carbon atoms to wave in and out of different hybrid states (sp³, sp², and sp), shaping the structure and reactivity of organic compounds. This plays a fundamental role in the chemistry of life, wherein carbon is the backbone of amino acids, nucleotides, and carbohydrates—all essential components for biological processes.
5.3 Future Research Directions in Electron Configuration Studies
Looking forward, ongoing research in electron configurations may lead to advancements in materials science, nanotechnology, and quantum computing. Understanding electron configurations at the atomic and sub-atomic levels could pave the way for designing new materials with tailored properties, enhanced catalytic systems, and even insights into artificial intelligence at the molecular level.