1. Introduction to the Electron Configuration of C
The study of electron configuration plays a pivotal role in understanding the behavior of atoms, especially in the context of chemistry and physics. In this comprehensive guide, we delve into the electron configuration of carbon (C), a fundamental element in organic chemistry and life itself. By exploring its electron arrangement, we can gain insights into its unique properties and how it forms bonds with other elements.
Before we dive deeper into carbon’s electron configuration, let’s first clarify the concept of electron configuration itself. You will learn not only the notation used but also the significance of carbon in a broader chemical context. For a detailed breakdown, you can refer to electron configuration of c.
1.1 What is Electron Configuration?
Electron configuration refers to the distribution of electrons in an atom’s energy levels or orbitals. It provides a specific notation that scientists use to convey how the electrons are arranged around the nucleus of an atom. The configuration is typically represented using a series of numbers and letters that denote the energy levels (shells) and subshells that contain electrons.
For example, the configuration of an atom can be written in the form of 1s², 2s², 2p⁶, etc., where the number represents the energy level, and the letters (s, p, d, f) correspond to the type of orbital. The superscript indicates the number of electrons in each subshell.
1.2 Importance of Carbon in Chemistry
Carbon is often referred to as the backbone of life due to its unique ability to form stable bonds with a variety of elements, including itself. This capacity allows for the vast diversity of organic compounds, which play critical roles in biological processes. From carbohydrates to complex proteins, carbon’s versatility makes it central to biochemistry and organic chemistry.
The atomic number of carbon is 6, which means it has six protons and, in its neutral state, six electrons. The arrangement of these electrons dictates how carbon interacts with other elements and the types of bonds it can form. Understanding carbon’s electron configuration is therefore essential for predicting its chemical behavior.
1.3 Overview of Carbon’s Electron Configuration
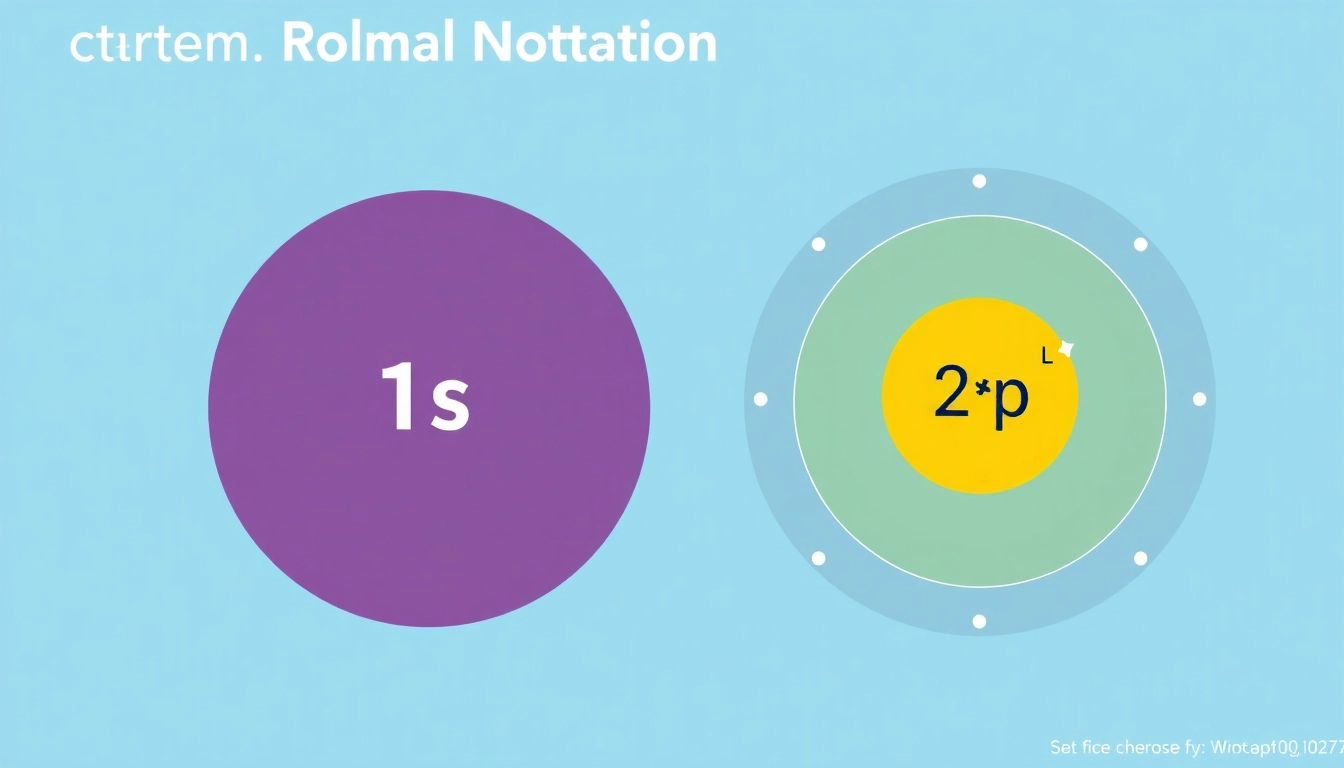
The electron configuration of carbon in its ground state is 1s² 2s² 2p². This notation indicates that there are two electrons in the first shell (1s), two in the second shell’s s subshell (2s), and two in the second shell’s p subshell (2p). This distribution affects carbon’s bonding capabilities and reactivity.
In comparison to other elements, carbon’s specific electron configuration allows it to form four covalent bonds, leading to a wide variety of molecules and complex structures. It also plays a crucial role in phenomena such as hybridization and resonance in organic compounds.
2. The Basics of Electron Configuration for Carbon
2.1 How to Write the Electron Configuration of C
Writing the electron configuration for carbon follows the Aufbau principle, which states that electrons occupy the lowest energy orbitals first. Given its atomic number of six, we apply the following steps:
- Start from the lowest energy level (1s). Fill it with up to two electrons.
- Next, move to the second energy level (2s) and fill it with another two electrons.
- Finally, fill the 2p orbital with the remaining two electrons.
The resulting configuration, as noted earlier, is 1s² 2s² 2p². Each of these subshells can hold a certain maximum number of electrons, which ensures that during configuration, electrons are maximized in terms of energy efficiency.
2.2 Orbital Diagrams: Visual Representation
To better understand electron configurations, it is helpful to use orbital diagrams. These diagrams visually represent the arrangement of electrons in an atom’s orbitals. Each type of orbital—s, p, d, and f—has a specific shape and orientation:
- s orbitals: Spherical shape, can hold a maximum of 2 electrons.
- p orbitals: Dumbbell-shaped, can hold a maximum of 6 electrons (2 in each of the three orientations: px, py, pz).
- d orbitals: More complex shapes, can hold a maximum of 10 electrons.
- f orbitals: Even more complex shapes, can hold a maximum of 14 electrons.
For carbon, the 1s and 2s orbitals are fully filled with two electrons each, while the 2p orbital has two electrons, which can be represented as follows:
1s: ↑↓
2s: ↑↓
2p: ↑ ↑
This configuration highlights how the 2p subshell is half-filled, indicating that carbon can form covalent bonds with other atoms by sharing or exchanging electrons.
2.3 Common Mistakes in Writing Carbon’s Configuration
When writing the electron configuration for carbon, there are common pitfalls that students and chemists alike may encounter:
- Forgetting the Aufbau principle: It’s crucial to remember to fill lower energy levels before moving to higher ones.
- Neglecting to adhere to the Pauli exclusion principle: No two electrons can have the same set of four quantum numbers, which means that in the same orbital, electrons must have opposite spins.
- Confusion with noble gas shorthand: While the shorthand notation can simplify writing configurations, it is essential to understand the complete configuration of the element to avoid mistakes.
To avoid these mistakes, practice writing electron configurations alongside utilizing visual aids like orbital diagrams.
3. Significance of Carbon’s Electron Configuration
3.1 Chemical Bonds and Reactivity
Carbon’s electron configuration directly influences its ability to form chemical bonds. With four valence electrons in its outer shell (the 2s and 2p orbitals), carbon is tetravalent, meaning it can form four covalent bonds with other atoms. This property is a cornerstone of organic chemistry, as carbon can bond to a wide variety of elements, including hydrogen, oxygen, nitrogen, and even other carbon atoms.
Considering its reactivity, carbon can participate in different types of bonding, including:
- Single Bonds: Formed when two atoms share one pair of electrons (e.g., in methane, CH₄).
- Double Bonds: Formed by sharing two pairs of electrons (e.g., in ethylene, C₂H₄).
- Triple Bonds: Involves three pairs of shared electrons (e.g., in acetylene, C₂H₂).
This versatility allows for the formation of various complex molecules, from simple hydrocarbons to intricate proteins and nucleic acids.
3.2 The Role in Organic Molecules
The unique ability of carbon to form long chains and rings makes it essential in the study of organic molecules. These structures can exhibit a variety of functional groups that determine the chemical properties of the compounds. The importance of carbon compounds in biochemistry cannot be overstated—biomolecules such as carbohydrates, lipids, proteins, and nucleic acids are all fundamentally carbon-based.
For example, the structure of glucose, a key energy source for living organisms, features a ring structure with multiple hydroxyl (-OH) groups, all of which stem from carbon’s ability to form diverse bonds. Each connection illustrates how the arrangement of electrons, as described by its electron configuration, influences the behavior of the entire molecule.
3.3 Isotopes and Different Forms of Carbon
Carbon has several isotopes, which are variants of an element that have the same number of protons but different numbers of neutrons. The most common isotopes of carbon are Carbon-12 (¹²C) and Carbon-14 (¹⁴C). The electron configuration for these isotopes remains the same as the stable carbon atom, 1s² 2s² 2p².
However, the different mass numbers lead to varying stability and radioactivity properties. For instance, Carbon-14 is radioactive and is used in radiocarbon dating, a technique utilized in archaeology and geology to determine the age of ancient organic materials.
The presence of these isotopes further emphasizes the intricate balance of carbon’s chemistry and how its electron configuration dictates the properties of different isotopes, impacting their applications in science.
4. Advanced Concepts: Excited States and Electron Configuration
4.1 Differences in Electron Configuration when Excited
Atoms can absorb energy, which may cause electrons to jump to higher energy levels, resulting in an excited state. For carbon, this means that instead of its typical ground state configuration (1s² 2s² 2p²), electrons may occupy higher energy orbitals. This transition can result in configurations such as 1s² 2s¹ 2p³, where one electron has moved from the 2s to the 2p orbital.
These excited states can significantly affect chemical reactivity. For example, when carbon is in an excited state, its ability to bond can change, potentially enabling it to engage in different pathways to form new compounds.
4.2 Understanding Hybridization in Carbon
Hybridization is a key concept that emerges from carbon’s electron configuration, particularly concerning its ability to form four equivalent covalent bonds in organic molecules. Through hybridization, the atomic orbitals of carbon can mix to form new orbitals called hybrid orbitals.
Common hybridization states for carbon include:
- sp³ Hybridization: Forms four sigma bonds, as seen in methane (CH₄).
- sp² Hybridization: Forms three sigma bonds and one pi bond, characteristic of alkenes (e.g., C₂H₄).
- sp Hybridization: Leads to two sigma bonds and two pi bonds, present in alkynes (e.g., C₂H₂).
This hybridization allows carbon to adapt its bonding characteristics to create various molecular geometries and promote diverse chemical reactions, which is central to organic chemistry’s complexity.
4.3 Implications for Chemical Behavior
The electron configuration of carbon, along with its potential for hybridization, imparts distinct chemical behaviors that are crucial for understanding reactions in organic chemistry. Considerations include:
- Reactivity: The presence of unpaired electrons in carbon’s electron configuration indicates its propensity to form bonds.
- Stability: The structure and stability of organic compounds depend on how well the electron configuration facilitates bonding.
- Geometric Isomerism: Due to the varied bonding angles created by hybridization, carbon compounds can exhibit different geometric forms, affecting their physical and chemical properties.
Ultimately, understanding carbon’s electron configuration leads to valuable insights into its role in larger chemical systems, particularly in fields such as medicinal chemistry, materials science, and biochemistry.
5. Conclusion and Key Takeaways
5.1 Recap of Carbon’s Electron Configuration
In summary, carbon’s electron configuration, represented as 1s² 2s² 2p², is fundamental to its identity and behavior as a building block of life. By following the principles of electron arrangement and understanding common pitfalls, we are better equipped to explore the chemical properties that define carbon and its interactions with other elements.
5.2 Future Trends in Carbon Chemistry
As the field of chemistry continues to evolve, the versatility of carbon will likely see applications in emerging technologies such as organic photovoltaics and drug development. As researchers uncover the subtleties of carbon’s behavior at the molecular level, the potential to innovate within material science and nanotechnology becomes increasingly tangible.
5.3 Additional Resources for Further Learning
Those interested in delving deeper into the world of electron configurations and carbon chemistry can explore various educational resources, including academic journals, books on organic chemistry, and credible online platforms. Engaging with experts in the field through conferences and seminars can also provide valuable insights into cutting-edge research.