1. Introduction to Electron Configuration
What is Electron Configuration?
Electron configuration refers to the distribution of electrons in an atom’s orbitals. These orbitals are defined by quantum mechanics and are important for determining how atoms interact with one another. In a simplified sense, the configuration shows in which energy level and sublevel the electrons are located. For instance, the electron configuration of a carbon atom reveals how its six electrons are arranged, which plays a crucial role in its chemical behavior.
Importance of Electron Configuration in Chemistry
The electron configuration of an atom is fundamental for several reasons. Firstly, it dictates the atom’s chemical properties, including how it bonds with other atoms to form molecules. Additionally, understanding electron configurations is crucial for predicting the reactivity of an element and its placement in the periodic table. This understanding helps chemists design new materials, comprehend biological processes, and develop pharmaceuticals.
Overview of Carbon Atom Characteristics
Carbon is one of the most essential elements for life, with an atomic number of 6. It is a non-metal, characterized by its ability to form covalent bonds with other elements. Carbon atoms can bond in various structural configurations, including chains and rings, owing to their four valence electrons. This property makes carbon a cornerstone of organic chemistry, as it lays the foundation for the vast diversity of biological molecules.
2. Basics of Electron Configuration
Key Principles of Electron Configuration
Understanding electron configurations begins with a grasp of key principles such as the Pauli Exclusion Principle, Hund’s Rule, and the Aufbau Principle:
- Pauli Exclusion Principle: No two electrons can have the same set of four quantum numbers within an atom, which translates to no more than two electrons occupying the same orbital.
- Hund’s Rule: Within a sublevel, electrons will first fill degenerate orbitals (orbitals of equal energy) singly before pairing up. This minimization of electron-electron repulsion results in a more stable configuration.
- Aufbau Principle: Electrons fill atomic orbitals starting from the lowest energy level to the highest in a specific order, defined by the 1s, 2s, 2p, etc.
Understanding Atomic Orbitals
Atomic orbitals are the regions in which electrons are likely to be found around the nucleus. They are categorized into different shapes, such as s, p, d, and f. Each type of orbital has a specific energy level:
- s-orbitals: Spherical shape, each can hold up to two electrons.
- p-orbitals: Dumbbell-shaped, can hold up to six electrons in three different orientations.
- d-orbitals: More complex shapes, with five available orientations allowing for ten electrons.
- f-orbitals: Even more complex, accommodating fourteen electrons across seven different orientations.
Electron Configuration Notation Explained
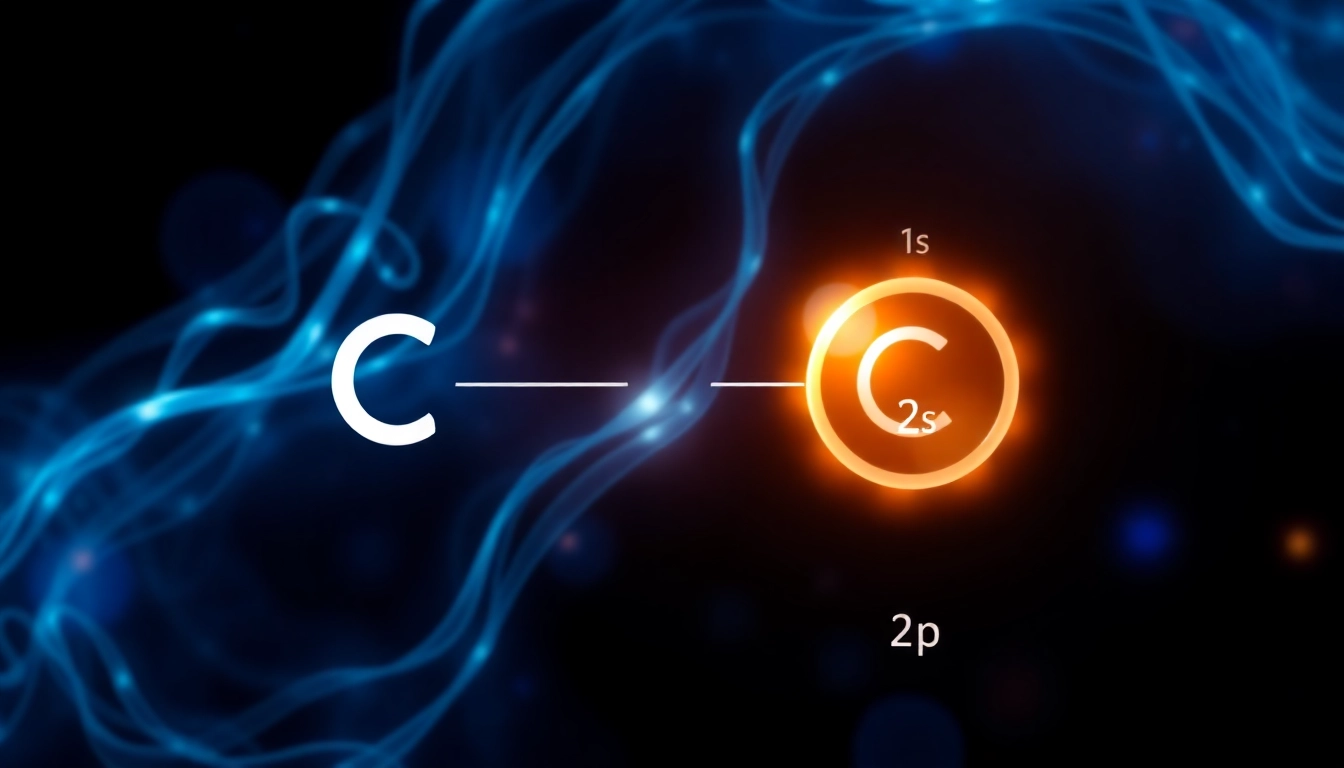
Electron configuration is denoted using a sequence of numbers and letters. The number indicates the principal energy level, while the letter represents the type of orbital. The superscript denotes how many electrons are present in those orbitals. For example, the electron configuration for carbon is represented as 1s2 2s2 2p2, meaning it has two electrons in the 1s orbital, two in the 2s orbital, and two in the 2p orbital.
3. Electron Configuration of a Carbon Atom
The Ground State Configuration
A neutral carbon atom has six electrons. According to the Aufbau principle, these electrons fill the orbitals in the following order: first the 1s orbital, then the 2s orbital, and finally the 2p orbitals. Thus, the electron configuration of a carbon atom in its ground state is 1s2 2s2 2p2, which means:
- 2 electrons in the 1s orbital.
- 2 electrons in the 2s orbital.
- 2 electrons in the 2p orbital.
Visual Representation of Carbon’s Electron Configuration
To visualize the electron configuration of carbon, it is helpful to refer to an orbital diagram. In this diagram, the orbitals are represented as boxes for the 1s, 2s, and 2p orbitals. Each box is filled according to the rules mentioned earlier:
1s|↑↓
2s|↑↓
2p|↑↓↑
This diagram simplifies understanding how carbon’s electrons are distributed among its atomic orbitals.
Comparing Carbon’s Configuration with Other Elements
Carbon’s electron configuration can be compared with elements from different groups in the periodic table to illustrate its unique properties. For example:
- Boron (B): Has one less electron than carbon, resulting in a configuration of 1s2 2s2 2p1. This indicates a different chemical reactivity, allowing boron to form different types of compounds.
- Nitrogen (N): Has one more electron than carbon, with a configuration of 1s2 2s2 2p3. Nitrogen’s reactivity and bonding capabilities reflect its additional electron and the resulting ability to form triple bonds.
- Oxygen (O): Exhibits a configuration of 1s2 2s2 2p4, leading to the formation of strong covalent bonds and its vital role in biological systems.
4. Implications of Carbon’s Electron Configuration
How It Affects Chemical Bonding
Carbon’s ability to form four covalent bonds due to its four valence electrons is a critical feature highlighted by its electron configuration. The hybridization of these electrons leads to the formation of various shapes and structures of molecules. For instance, in methane (CH4), carbon undergoes sp3 hybridization to form four equivalent bonds, creating a tetrahedral geometry.
Carbon’s Role in Organic Chemistry
Carbon is the backbone of organic chemistry because of its unique ability to form stable bonds with many elements, including itself. The various hybridizations (sp, sp2, and sp3) that carbon can undergo facilitate the creation of a spectrum of organic molecules such as hydrocarbons, alcohols, and carboxylic acids. This versatility allows organic compounds to perform a multitude of functions in biological systems.
Applications in Real-world Chemistry
The implications of carbon’s electron configuration extend beyond theoretical frameworks. In materials science, carbon allotropes such as graphite and diamond exhibit vastly different properties due to their unique bonding arrangements. Moreover, carbon compounds are crucial in pharmaceuticals, where specific configurations lead to desired therapeutic effects. Thus, understanding the electron configuration of carbon allows scientists to manipulate these compounds for various applications.
5. Advanced Concepts in Electron Configuration
Excited States of Electrons
When atoms absorb energy, electrons can be promoted to higher energy levels, resulting in excited states. In carbon, if one of its electrons in the 2p orbital absorbs energy, it may transition to a higher orbital, which can influence the atom’s reactivity and bonding capabilities. For instance, excited carbon atoms can participate in unique chemical reactions not available to their ground state counterparts.
Trends in Electron Configuration Across the Periodic Table
As we move across a period or down a group in the periodic table, trends in electron configuration become apparent. Generally, elements within the same group share similar valence electron configurations, leading to analogous chemical behaviors. For example, all group 14 elements (including carbon) will have four electrons in their outermost shell, while group 15 elements will have five.
Challenges and Common Misunderstandings
One common misunderstanding regarding electron configurations is the notion that they are purely a numerical representation without experimental implications. In contrast, the arrangement of electrons critically influences chemical properties, molecular structures, and the behavior of matter at a fundamental level. Another challenge is recognizing that electron configurations can change under different circumstances, such as ionization or chemical reactions, which can lead to confusion in predicting reactivity.