Introduction to Electron Configuration for Carbon
The study of electron configurations plays a pivotal role in understanding the chemical properties of elements. Among these, the electron configuration of carbon is particularly critical because of carbon’s fundamental role in organic chemistry and its significance in a wide array of scientific fields. If you’ve ever wondered what is the electron configuration for carbon?, you’re headed in the right direction to uncover the intricate mechanisms that shape chemical bonding and molecular behavior. This article provides a comprehensive overview of carbon’s electron configuration, detailing the foundational principles, the process of writing it, and its applications in various scientific disciplines.
Defining Electron Configuration
Electron configuration refers to the distribution of electrons in an atom’s electron shells and subshells. It dictates how electrons occupy various orbitals, which is crucial for understanding an element’s reactivity and bonding characteristics. The electron configuration offers a roadmap detailing where each electron resides and helps in predicting how atoms will interact with one another.
Importance of Carbon in Chemistry
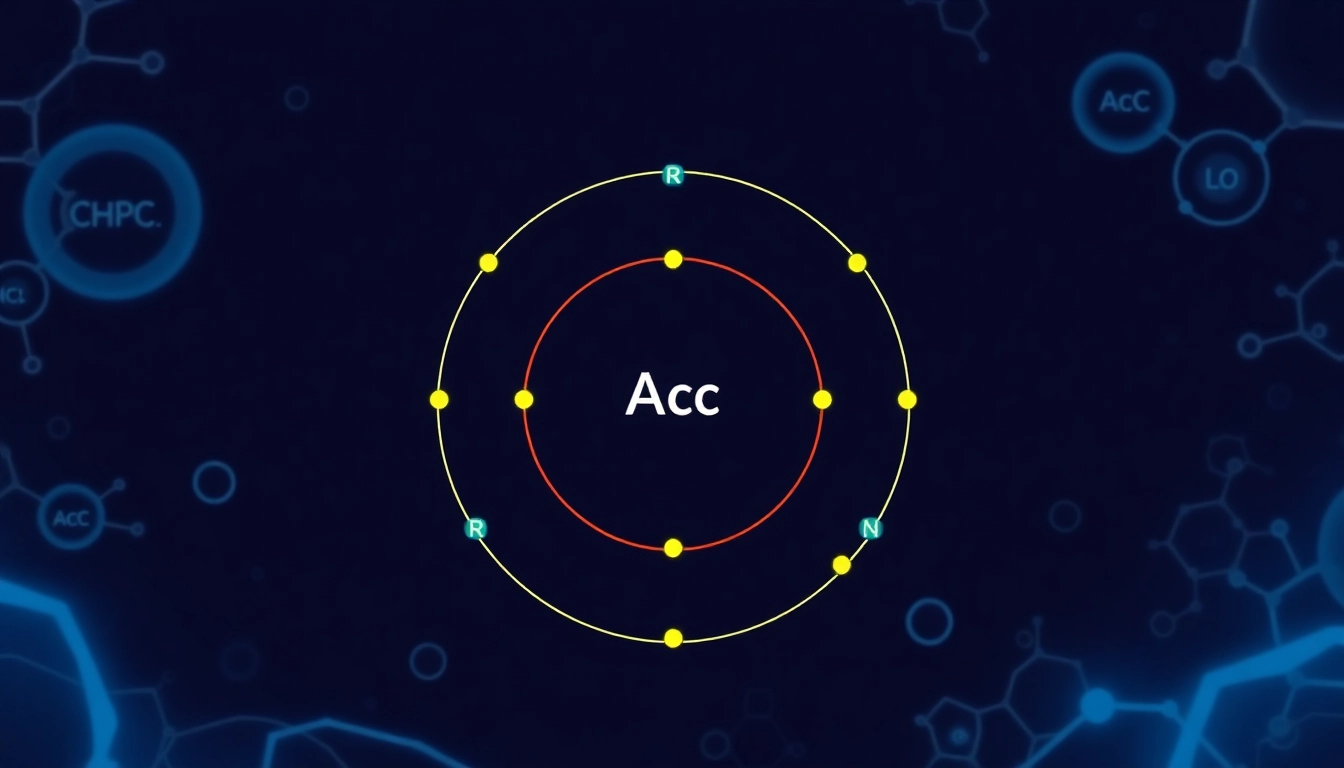
Carbon, with an atomic number of 6, is unique in its ability to form stable bonds with many elements, including itself. This property facilitates the formation of a vast array of organic compounds, laying the foundation for life on Earth. The versatility and robustness of carbon’s chemistry are largely influenced by its electron configuration—1s² 2s² 2p²—which allows it to engage in covalent bonding to form complex molecules. Understanding carbon’s electron arrangement thus embodies a core concept in chemistry.
Overview of Electron Orbitals
Electron orbitals are regions around the nucleus of an atom where electrons are likely to be found. Each orbital can hold a maximum of two electrons with opposite spins. The designated shapes and energy levels of these orbitals affect the geometry of molecules and their reactivity. The four types of orbitals are s, p, d, and f, with the s and p orbitals being most relevant for carbon. The s orbital can hold 2 electrons, while the p orbitals can accommodate a total of 6 electrons, allowing a rich interplay of bonding configurations.
How to Write the Electron Configuration for Carbon
Step-by-Step Process
Writing the electron configuration for carbon involves a systematic approach, typically following the order of increasing energy levels (from low to high). The six electrons in a neutral carbon atom are distributed across the orbitals as follows:
- The first two electrons fill the 1s orbital.
- The next two electrons fill the 2s orbital.
- The remaining two electrons occupy the 2p orbitals.
Thus, the complete electron configuration for carbon can be expressed as: 1s² 2s² 2p².
Using the Aufbau Principle
The Aufbau principle, which means “building up” in German, plays a crucial role in determining electron configurations. According to this principle, electrons occupy the lowest energy orbitals first before filling higher-level orbitals. For carbon, the configuration is derived by placing the electrons within the orbitals starting from 1s before proceeding to 2s and then filling 2p. This approach underscores the predictability of electron placement and helps in visualizing chemical behavior.
Common Mistakes to Avoid
When writing the electron configuration, there are common pitfalls to be mindful of:
- Forgetting Electron Count: Ensure that the total number of electrons matches the atomic number of carbon, which is 6.
- Incorrect Order of Filling: Adhere strictly to the Aufbau principle; otherwise, you might misplace electrons.
- Not Considering Spin Directions: While filling orbitals, apply Hund’s Rule to ensure that each orbital in a subshell is singly occupied before pairing up electrons.
Electron Configuration Notation Explained
Full vs. Condensed Notation
The full electron configuration for carbon is expressed as 1s² 2s² 2p², indicating every individual electron’s position. However, condensed notation simplifies the expression. The condensed form reflects the electron configuration starting from the last noble gas prior to carbon, which is Helium (He in the case of carbon). Thus, the condensed notation becomes [He] 2s² 2p², allowing for more efficient and less cumbersome communication in chemistry.
Interpreting Orbital Diagrams
Orbital diagrams provide a visual representation of electron configurations, mapping out the arrangement of electrons in orbitals. For carbon, the orbital diagram would illustrate the 1s orbital with two electrons, the 2s orbital with two electrons, and the 2p orbitals with the last two electrons distributed among them. This representation aids in grasping concepts like hybridization and molecular geometry, key factors in understanding chemical reactions and bonding.
Examples of Other Elements
Comparative analysis of electron configurations reveals unique patterns among elements. For example, oxygen (O) has an electron configuration of 1s² 2s² 2p⁴, resulting in a propensity to form two bonds due to its six valence electrons. Meanwhile, nitrogen (N) possesses a configuration of 1s² 2s² 2p³, allowing it to engage in three bonds, showcasing the nuanced connectivity dictated by electron configuration across different elements.
Applications of Carbon’s Electron Configuration
Understanding Bonding and Hybridization
Carbon’s electron configuration plays a crucial role in hybridization—an essential concept in molecular chemistry. The ability of carbon to hybridize its orbitals (such as forming sp³, sp², and sp hybridizations) directly impacts molecular shape and bond angles. For instance, tetrahedral geometry arises when carbon makes four equivalent sp³ hybrid bonds, as seen in methane (CH₄). This flexibility enables carbon compounds to adopt diverse structures, impacting their physical and chemical properties.
Reactivity of Carbon and its Compounds
Carbon’s reactivity is intricately linked to its electron configuration, influencing its ability to form various compounds. The arrangement of electrons determines the types of bonds carbon can form (single, double, and triple bonds), impacting the stability and reactivity of organic molecules. For instance, in organic chemistry, carbon compounds often participate in nucleophilic and electrophilic reactions, a behavior directly tied to electron distribution.
Carbon’s Role in Organic Chemistry
In organic chemistry, carbon stands out as the backbone element, forming its unique bond structures that are often complex and versatile. The electron configuration of carbon allows it to form long chains and rings, engaging in structural isomerism and stereoisomerism that generates immense diversity among organic compounds. This characteristic underlies virtually all biological pathways and the synthesis of synthetic materials, emphasizing the importance of understanding its electron configuration.
Conclusion and Further Research
Summary of Key Points
Understanding the electron configuration for carbon—1s² 2s² 2p² or condensed as [He] 2s² 2p²—is fundamental to grasping broader chemical concepts. This configuration not only describes how carbon operates independently but serves as a keystone for understanding its interactions and compounds.
Resources for Learning More
For those interested in delving deeper into the topic of electron configurations and related chemistry concepts, the following resources may be useful:
- Electron Configuration for Carbon (C) – TerpConnect
- 2.2: Electron Configurations – Chemistry LibreTexts
- Understanding Electron Configuration Questions – Chegg
FAQs About Electron Configuration
As you navigate the intricacies of electron configurations, you may encounter additional questions:
- What happens if an atom gains or loses electrons? Such changes will affect its electron configuration and can lead to the formation of ions.
- How does electron configuration influence an atom’s reactivity? The arrangement of electrons determines how an atom interacts with others, influencing its chemical properties and reactions.
- Can electron configurations predict the state of matter of an element? While not definitive, the electron configuration can provide insights into an element’s phase at given conditions (solid, liquid, gas) based on its bonding characteristics.