Introduction to Electron Configuration
Understanding electron configuration is essential for grasping the fundamentals of chemistry. Specifically, when we give the electronic configuration for carbon, we are delving into the arrangement of electrons around the nucleus of a carbon atom, which plays a pivotal role in its chemical behavior and bonding capabilities.
Definition of Electron Configuration
Electron configuration refers to the distribution of electrons in an atom’s atomic orbitals. Each electron in an atom occupies a specific energy level and sublevel. In essence, it is a way for chemists to describe how electrons are arranged around the nucleus of an atom, thereby providing valuable insights into an element’s reactivity and bonding characteristics.
Importance of Electron Configuration in Chemistry
The electron configuration of an element holds profound significance in chemistry. It helps predict how an atom will interact with other atoms and the types of bonds it can form. For instance, elements with similar electron configurations typically exhibit similar chemical properties. Moreover, understanding electron configurations is crucial in fields like organic chemistry, materials science, and biochemistry, where the behavior and properties of molecules are directly influenced by electron arrangements.
Overview of Carbon’s Atomic Structure
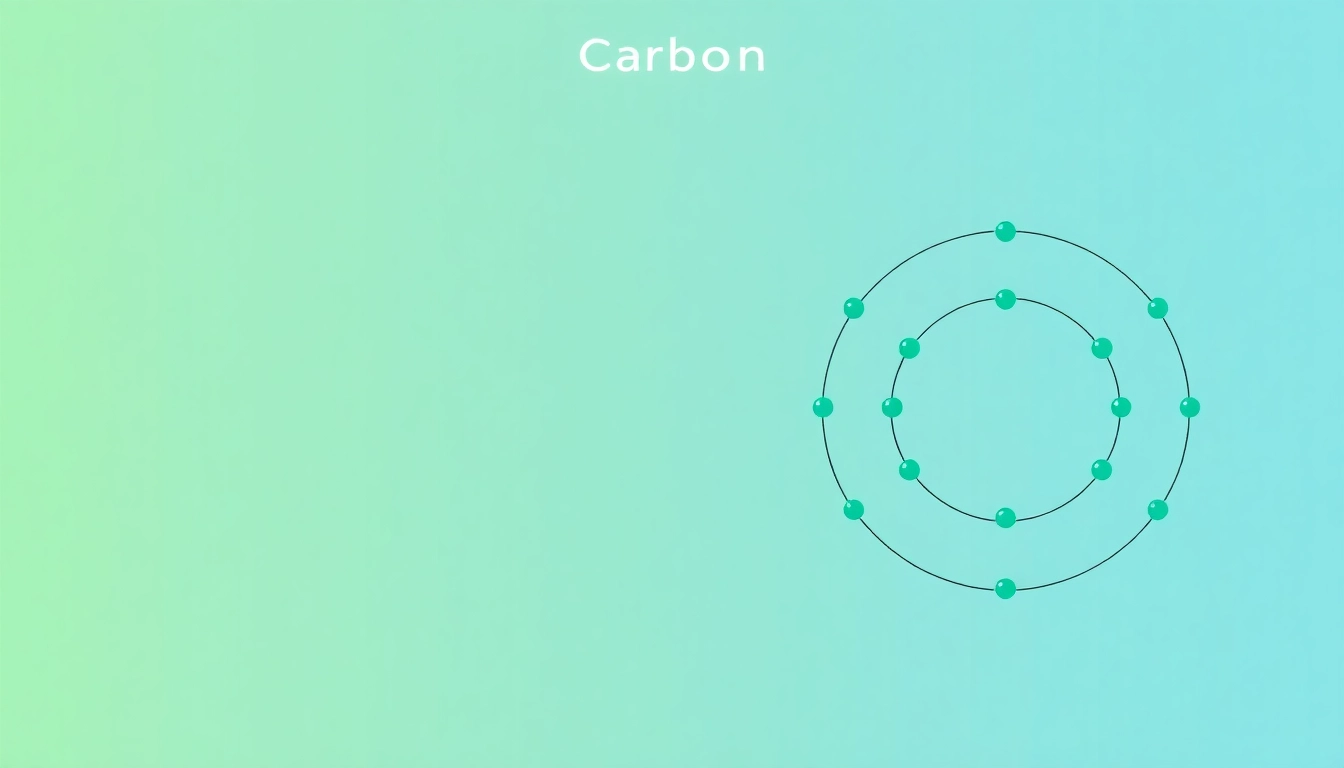
Carbon, denoted by the symbol “C” and possessing an atomic number of 6, has a unique position in the periodic table. Its small atomic size and ability to form four covalent bonds make it an unparalleled element in terms of its versatility and role in the chemistry of life. The ground state electron configuration for carbon is 1s2 2s2 2p2, indicating that it has six electrons distributed in various orbitals.
Step-by-Step Guide to Give the Electronic Configuration for Carbon
Understanding Electron Shells and Orbitals
To write the electronic configuration for an element, one must first understand the concept of electron shells and orbitals. Electrons are arranged in energy levels called shells, which are numbered 1, 2, 3, and so on. Each shell contains subshells (s, p, d, f), which further divide into orbitals where electrons reside. The general order of filling these orbitals follows the Aufbau principle, which states that electrons will fill lower-energy orbitals before moving to higher-energy orbitals.
Writing the Electronic Configuration for Ground State
For a neutral carbon atom, which has an atomic number of 6, the process of writing its ground state electron configuration is fairly straightforward:
- The first two electrons occupy the 1s orbital. Thus, we write 1s2.
- The next two electrons fill the 2s orbital: 2s2.
- The remaining two electrons go into the 2p orbital, leading to 2p2.
As a result, the complete ground state electron configuration for carbon is 1s2 2s2 2p2.
Exploring Excited States and Anomalies
While the ground state electron configuration is crucial for defining an element’s basic properties, electrons can also be excited to higher energy levels through absorption of energy. For carbon, an excited state configuration might rearrange the electrons, such as promoting one electron from the 2s orbital to the 2p orbital, resulting in configurations like 1s2 2s1 2p3. This excited state can lead to different chemical behaviors and bonding properties.
Common Questions About Carbon’s Electron Configuration
How to Write Electron Configuration for Ions
When carbon forms ions, its electron configuration changes. For example, when carbon loses an electron to form a cation (C+), it loses one from the 2p orbital, becoming 1s2 2s2 2p1. Conversely, when it gains an electron to form an anion (C−), it gains an additional electron in the 2p orbital, resulting in 1s2 2s2 2p3.
Comparison with Other Elements
Carbon’s electron configuration can be compared with other elements to highlight its unique bonding capabilities. For instance, oxygen (O) with the configuration 1s2 2s2 2p4 can form two bonds, while nitrogen (N) with 1s2 2s2 2p3 can form three bonds. These comparisons elucidate why carbon is often called the backbone of organic chemistry, as it can form four stable covalent bonds and create diverse molecular frameworks.
Common Misunderstandings
One common misunderstanding regarding electron configuration is related to the ability of carbon to form multiple bonds due to its flexibility in bonding. Many students incorrectly think that carbon can only form four single bonds. However, carbon can participate in double and triple bonds, affecting the structure and reactivity of organic molecules significantly. Understanding carbon’s electron configuration is crucial to grasp its versatility in forming stable compounds.
Applications of Electron Configuration in Real Life
Role in Chemical Bonding and Reactivity
Electron configuration fundamentally dictates how atoms interact and bond with each other. The arrangement of carbon’s electrons allows it to participate in a variety of chemical reactions, forming single, double, and triple bonds. This versatility is not only essential in organic chemistry but also in the development of new materials, pharmaceuticals, and biochemicals, owing to the myriad of structures that carbon can help create.
Electron Configuration and Organic Chemistry
Within organic chemistry, the electron configuration of carbon underpins the formation of hydrocarbons and complex organic molecules. Understanding how carbon’s electrons are arranged leads to predicting its behavior in reactions and its ability to form various functional groups. This knowledge is particularly useful in the synthesis of drugs, polymers, and bioactive molecules, where tailored carbon configurations yield specific functionalities.
Educational Importance and Curriculum Insights
In an educational context, electron configuration plays a pivotal role throughout chemistry curricula. Teaching students about carbon’s electron configuration equips them with essential tools for understanding concepts such as hybridization, resonance, molecular geometry, and reactivity patterns. These concepts are foundational for future studies in chemistry, biology, and materials science. Moreover, students’ grasp of electron configuration aids in mastering more complex topics, making it a critical area of focus in science education.
Conclusion and Further Reading
Summarizing Carbon’s Electron Configuration
In summary, the electron configuration of carbon, which is 1s2 2s2 2p2, is foundational for understanding its chemical behavior, bonding capabilities, and overall importance in the field of chemistry. By examining how carbon’s electrons are arranged, we gain insights into the reactivity and properties of carbon-containing compounds, which are central to many scientific and industrial applications.
Resources for Further Study
For those looking to deepen their understanding of electron configurations and their implications in chemistry, a variety of resources are available. Textbooks on general chemistry, online courses, and academic journals offer extensive coverage of atomic theory and electron behavior. Websites like Khan Academy and Coursera also provide structured learning paths for students and professionals alike.
Encouragement to Explore Chemistry Further
The world of chemistry is vast and exciting, with new discoveries emerging regularly. By understanding the fundamental concept of electron configuration, you take a significant step into a realm filled with potential for innovation and discovery. I encourage readers to continue exploring this fascinating field, ensuring a deeper grasp of the elements that make up our universe.