Introduction to Carbon and Its Electron Configuration
Carbon is one of the most fundamental elements in the universe, playing a critical role in organic chemistry and the molecular structures that make up all life. The electron configuration of carbon provides insights into its chemical properties and behavior in reactions. Specifically, understanding the carbon electron shell offers foundational knowledge pertinent to various scientific disciplines, including biology, chemistry, and materials science. In this article, we will explore the structure of carbon’s electron shell, how it influences chemical behavior, and its applications across different scientific domains.
What is the Carbon Atom?
The carbon atom is a chemical element represented by the symbol C on the periodic table and has an atomic number of 6. This means that carbon contains six protons in its nucleus and, when neutral, six electrons that balance the positive charge of the protons. Carbon’s unique ability to form stable bonds with a variety of other elements is attributed to its atomic structure and electron configuration. The six electrons of carbon are arranged in specific orbitals and shells, crucial for the element’s reactivity and bonding behavior.
Electrons and Their Importance in Chemistry
Electrons are fundamental subatomic particles that carry a negative charge. They occupy orbitals around an atom’s nucleus and are responsible for chemical bonding and reactions. The distribution of electrons in an atom determines how it interacts with other atoms, leading to various chemical behaviors. This importance is particularly evident in elements like carbon, where the arrangement of electrons allows for the formation of stable compounds, including those vital for life, such as proteins, DNA, and carbohydrates.
Overview of Electron Configuration
Electron configuration refers to the distribution of electrons among the various orbitals of an atom. This configuration influences an atom’s chemical characteristics and reactivity. For carbon, the ground state electron configuration can be expressed as 1s² 2s² 2p². This notation indicates that carbon has two electrons in the first energy level (the 1s orbital) and four electrons in the second energy level (two in the 2s orbital and two in the 2p orbital). Understanding this configuration lays the groundwork for further discussing the carbon electron shell structure and its implications.
The Structure of the Carbon Electron Shell
Understanding Shell Levels
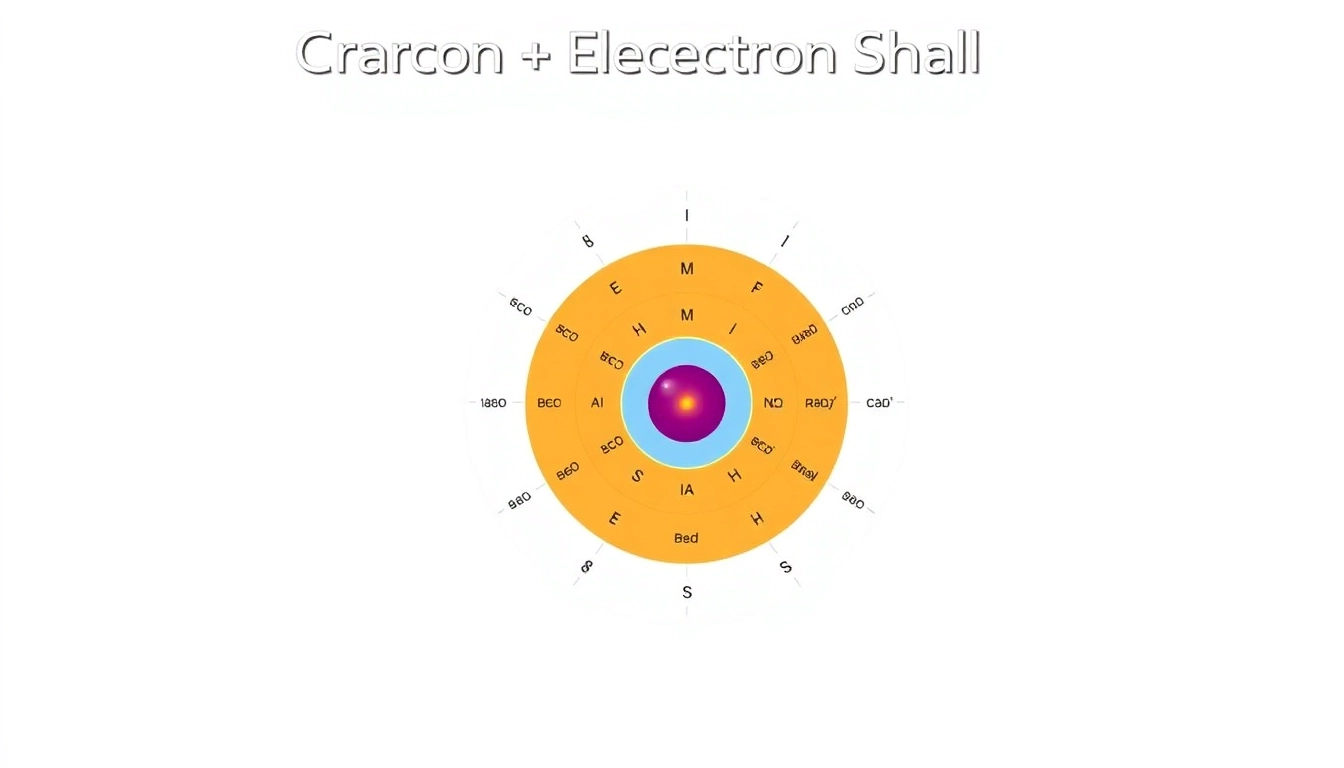
The electron shells of an atom are defined by the energy levels that electrons occupy. For carbon, the two electron shells accommodate a total of six electrons as follows:
- First Shell (K Shell): Can hold up to 2 electrons, which is filled in the case of carbon with both electrons occupying the 1s orbital.
- Second Shell (L Shell): Can hold up to 8 electrons but contains only 4 electrons in carbon, where 2 electrons are in the 2s orbital and 2 electrons are in the 2p orbitals.
This arrangement of electrons determines not only the stability of the carbon atom but also its ability to partake in chemical bonding and other interactions.
Electron Distribution in Carbon
As previously noted, carbon has a total of six electrons. The electron distribution can be succinctly described as follows:
- 1s Orbital: Contains 2 electrons, which are the innermost shell electrons.
- 2s Orbital: Contains 2 electrons, representing the first two outer shell electrons.
- 2p Orbitals: Contains 2 electrons, which are spread across the available p orbitals (there are three 2p orbitals, each able to hold a maximum of two electrons).
This specific arrangement highlights that carbon has four electrons in its outer shell, known as valence electrons, which play a crucial role in chemical bonding.
Visual Representation of the Carbon Electron Shell
Visualizing the electron shell structure aids in understanding carbon’s bonding capabilities. Typically, diagrams depicting the carbon atom illustrate:
- A nucleus at the center representing protons and neutrons.
- Concentric circles representing electron shells.
- Positions of electrons within the shells and orbitals, indicating their distribution and energy levels.
These representations illustrate how closely packed the 1s electrons are to the nucleus, while the 2s and 2p electrons are further away, leading to the unique bonding properties of carbon.
Detailed Analysis of Carbon’s Electron Shell Arrangement
Inner Shell Electrons: Role and Characteristics
In the context of the electron shell configuration of carbon, the inner shell consists of 2 electrons in the 1s orbital. This configuration is stable and does not participate directly in bonding. However, it is crucial because:
- It provides a foundational positive charge balance for the nucleus.
- It influences the energy levels of the outer valence electrons, stabilizing the atom.
The occupancy of the 1s orbital also sets the stage for the chemical reactivity of the outer shell electrons, where all chemical interactions take place.
Outer Shell Electrons and Their Chemical Behavior
With four electrons in the outer shell, carbon has unique chemical properties. These valence electrons occupy the 2s and 2p orbitals, allowing for a variety of bonding configurations:
- Single Covalent Bonds: Carbon can share one of its valence electrons with another atom, forming a stable covalent bond, as seen in methane (CH₄).
- Double and Triple Bonds: With its four valence electrons, carbon can also form double or triple bonds, as evident in ethylene (C₂H₄) and acetylene (C₂H₂).
- Hybridization: Carbon can undergo hybridization, where its s and p orbitals mix to form new hybrid orbitals, optimizing its bonding capability.
These behaviors are rooted in the electron configuration and arrangement of outer shell electrons, leading to carbon’s classification as a versatile building block for many organic molecules.
Valence Electrons: The Key to Bonding
Valence electrons are the outermost electrons involved in forming bonds with other atoms. The four valence electrons in carbon dictate its ability to form a wide range of structures:
- Carbon can bond with itself, leading to long carbon chains or rings, a key characteristic of organic chemistry.
- It can form stable bonds with other non-metals, such as hydrogen, oxygen, nitrogen, and others, resulting in diverse molecular formations.
- The ability of carbon to form four bonds—either single, double, or triple—makes it uniquely suited for creating complex organic compounds.
In fact, the tetravalency of carbon is central to the complexity of organic chemistry and biochemistry.
Applications of Carbon’s Electron Configuration in Science
Impact on Organic Chemistry
Carbon’s electron configuration has profound implications in organic chemistry, which is the branch of chemistry focused on the study of carbon-containing compounds. The four valence electrons allow carbon to:
- Form extensive networks of carbon atoms that constitute organic compounds, from simple molecules to complex macromolecules such as proteins and nucleic acids.
- Exhibit isomerism, where compounds with the same molecular formula can have different structures and properties due to different arrangements of carbon atoms.
- Participate in various types of chemical reactions, including substitution, addition, and elimination, critical for synthesizing new organic materials.
Ultimately, the unique attributes of carbon’s electron shell facilitate the vast diversity of organic molecules found in nature and synthesized in laboratories.
Significance in Biological Systems
Carbon is the backbone of life on Earth, primarily due to its versatile bonding characteristics. In biological systems:
- Carbon atoms form the structural frameworks of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids.
- The ability to form stable bonds with other elements (like hydrogen, oxygen, and nitrogen) allows for the complexity necessary for biochemical processes.
- Carbon forms covalent bonds in such a way that it can create molecules with specific shapes necessary for biological function, such as enzymes and receptor molecules.
The electron configuration of carbon truly underpins life by enabling the vast array of biological processes that define living organisms.
Innovations in Material Science
The unique properties of carbon and its electron configuration have led to significant advancements in material science. For example,:
- Graphene: A single layer of carbon atoms arranged in a two-dimensional honeycomb lattice that exhibits exceptional electrical and thermal conductivity.
- Carbon Nanotubes: Cylindrical nanostructures that have remarkable strength and electrical properties, making them valuable for nanotechnology applications.
- Composites: Carbon fibers are used in composite materials, enhancing strength-to-weight ratios in industries like aerospace and automotive.
The innovative applications stemming from carbon’s diverse electron configurations influence technology and engineering fields, driving new materials that are both functional and efficient.
Frequently Asked Questions about Carbon’s Electron Shell
What are the Electron Shells of Carbon?
As previously mentioned, carbon has a total of six electrons situated in two electron shells. The first shell, the K shell, holds 2 electrons (1s²), while the second shell, the L shell, accommodates the remaining 4 electrons, with 2 in the 2s orbital and 2 in the 2p orbitals (2s² 2p²). This structure provides for its unique chemical properties.
How Does the Electron Shell Relate to Carbon’s Properties?
The arrangement of electrons in carbon’s shells directly influences its valence and therefore, its chemical behavior. Valence electrons dictate how an atom can bond with other atoms. Given that carbon has four valence electrons, it can form four covalent bonds, establishing its categorization as a tetravalent element, essential for forming the complex molecules that contribute to life and chemical diversity.
Why is Understanding the Electron Shell Important?
Understanding carbon’s electron shell is crucial for a variety of reasons, including:
- It aids in predicting how carbon will behave in chemical reactions.
- It establishes the foundation for organic chemistry and impacts the study of biochemistry.
- Knowledge of electron configurations is fundamental to the development and innovation of new materials.
In essence, a clear comprehension of the electron shell and configuration of carbon allows scientists and researchers to harness its properties effectively across multiple fields.