1. What is Electron Configuration?
1.1 Definition and Importance
Electron configuration refers to the distribution of electrons within an atom’s orbitals. This arrangement is crucial for understanding chemical reactivity, bonding, and the physical properties of elements. Each element’s unique electron configuration is a key characteristic that helps define how it behaves chemically and physically. As we delve into the carbon complete electron configuration, we will explore its foundational role in chemistry.
1.2 The Role in Chemistry
In chemistry, the electron configuration determines how an atom interacts with other atoms. The number of electrons in the outermost shell—known as valence electrons—influences an element’s ability to bond with other elements. For example, noble gases like neon are chemically inert due to their filled outer shells, while elements like carbon are highly reactive due to their four valence electrons.
1.3 Basic Principles Explained
The principles governing electron configuration can be summarized as follows:
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy levels to the highest.
- Pauli Exclusion Principle: No two electrons can have the same set of quantum numbers, meaning an orbital can hold a maximum of two electrons with opposite spins.
- Hund’s Rule: Electrons will occupy degenerate orbitals (orbitals with the same energy level) singly before pairing up, to maximize stability.
2. Carbon: A Vital Element in Chemistry
2.1 Overview of Carbon’s Properties
Carbon, with the chemical symbol C and atomic number 6, is unique due to its ability to form diverse compounds. It is the basis of organic chemistry and exists in various allotropes, including graphite, diamond, and fullerenes. Carbon has a relatively low electronegativity of 2.55 on the Pauling scale, which indicates a modest tendency to attract electrons.
2.2 The Significance of Carbon in Life
Carbon is essential for life on Earth, serving as the backbone of biological molecules. Proteins, nucleic acids, carbohydrates, and lipids all have carbon as a fundamental component. Its versatile bonding capabilities allow the formation of long chains, rings, and complex three-dimensional structures, which are vital for biological functions.
2.3 Comparing Carbon with Other Elements
When comparing carbon with other elements in the same group, such as silicon and germanium, it becomes clear that carbon’s bonding versatility is exceptional. While silicon can form complex structures, its compounds tend to be less stable than those of carbon. The ability of carbon to form stable covalent bonds with many elements enhances its prevalence in organic chemistry.
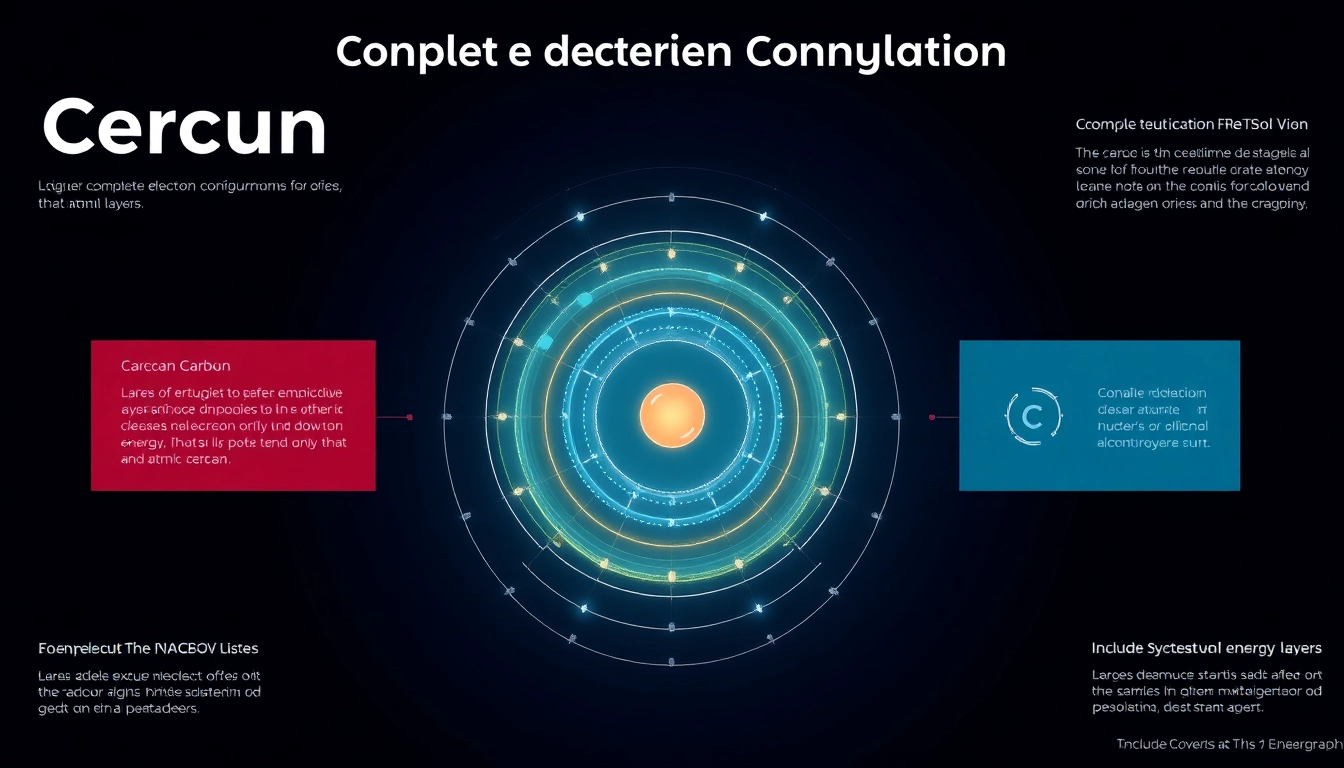
3. The Complete Electron Configuration of Carbon
3.1 Detailed Breakdown of 1s2 2s2 2p2
The complete electron configuration of carbon is expressed as 1s² 2s² 2p². This notation provides insight into how carbon’s six electrons are distributed:
- The 1s orbital can hold a maximum of two electrons, which carbon fills completely (1s²).
- The 2s orbital also holds two electrons (2s²).
- Finally, two electrons occupy the 2p subshell (2p²), allowing for the possibility of bonding and hybridization in various chemical reactions.
3.2 Visual Representation of Carbon’s Configuration
Visualizing the electron configuration is crucial for understanding how carbon interacts with other elements. The following orbitals can be diagrammatically represented:

This representation highlights the filled and partially filled orbitals of carbon, underlining its capacity for forming four bonds with other elements.
3.3 Common Misunderstandings in Electron Configurations
Oftentimes, misconceptions arise when interpreting electron configurations. One common misunderstanding is the belief that configurations like 1s² 2s² 2p² imply a lack of flexibility in bonding. However, carbon’s ability to hybridize—combining different orbitals to form equivalent energy levels—allows it to adapt its bonding patterns in molecular structures.
4. Using the Electron Configuration in Real-World Applications
4.1 Its Role in Chemical Bonding
The electron configuration is integral in determining the bonding characteristics of carbon. Carbon’s four valence electrons enable it to form stable covalent bonds through single, double, and even triple bonds. This flexibility is a cornerstone of organic chemistry and is manifested in molecular compounds such as methane (CH₄) and carbon dioxide (CO₂).
4.2 Applications in Material Science
In material science, carbon’s electron configuration allows for the creation of various forms of carbon-based materials, including nanomaterials, graphene, and carbon nanotubes. These materials exhibit remarkable strength, electrical conductivity, and thermal properties, driving advancements in electronics, energy storage, and nanotechnology.
4.3 Electron Configuration in Organic Chemistry
Understanding the electron configuration of carbon is essential for predicting organic reaction mechanisms and understanding the properties of organic compounds. For instance, the hybridization of carbon’s orbitals plays a crucial role in determining the shape of molecules, influencing their reactivity and interaction with biological systems.
5. Conclusion and Further Reading
5.1 Recap of Carbon’s Importance
Carbon is more than just an element; it is the foundation of life as we know it. Its unique electron configuration allows for diverse bonding patterns, making it central to organic chemistry and vital to life processes.
5.2 Suggested Resources for Deeper Learning
For those looking to deepen their understanding of carbon and its electron configuration, consider exploring the following resources:
- Electron Configuration for Carbon (C) – TerpConnect
- 2.2: Electron Configurations – Chemistry LibreTexts
- What is the electronic configuration of carbon? – Quora
5.3 Recent Research in Electron Configurations
Recent studies in quantum chemistry and material science continue to explore how variations in electron configurations can lead to new discoveries in catalysis, energy storage, and molecular technologies. Keeping abreast of these developments can provide invaluable insights into the future of chemistry and materials science.