Introduction to Electron Configuration
Understanding the electron configuration of elements is fundamental in the fields of chemistry and physics. Each element’s electronic structure not only influences its properties but also determines how it interacts with other elements. The electron configuration for carbon, a cornerstone of organic chemistry and biology, provides essential insights into its bonding behavior and chemical reactivity.
Definition and Importance
Electron configuration refers to the distribution of electrons in an atom’s atomic orbitals. It is critical for understanding the chemical behavior of the atom, its reactivity, and its position in the periodic table. For carbon, which has six electrons, its configuration explains its tetravalency and its ability to form various compounds crucial for life, including hydrocarbons and biomolecules.
Basic Terminology Explained
Key terms associated with electron configurations include:
- Atomic Number: The number of protons in an atom, which defines the element. Carbon has an atomic number of 6.
- Orbitals: Regions around the nucleus where electrons reside. Common types include s, p, d, and f orbitals.
- Valence Electrons: Electrons in the outermost shell that participate in bonding.
Overview of Quantum Mechanics Fundamentals
Electron configurations are based on principles of quantum mechanics, particularly the wave-particle duality of electrons. These principles govern how electrons occupy orbitals as they have quantized energies, meaning they can only exist in allowed energy levels.
Electron Configuration for Carbon: The Basics
Atomic Structure of Carbon
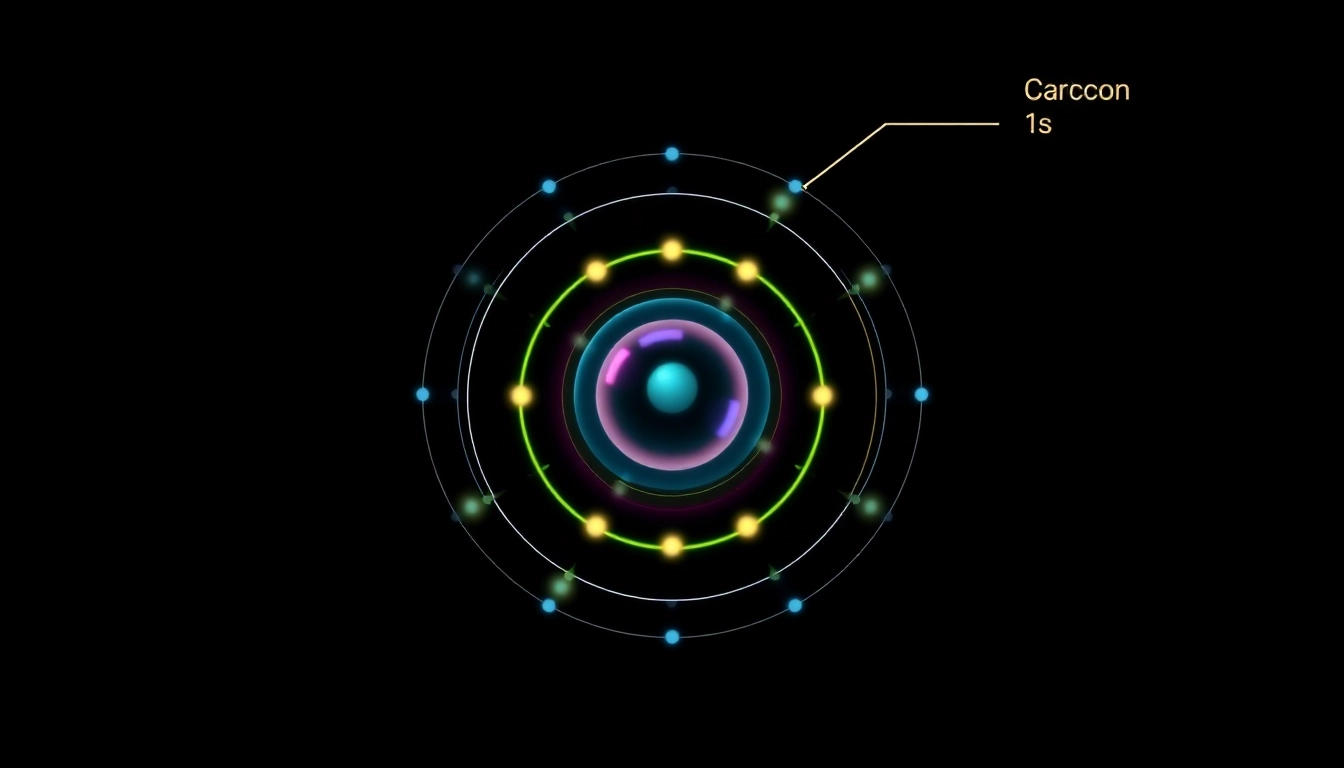
Carbon, with an atomic number of 6, contains six protons and, in its neutral state, six electrons. The arrangement of these electrons in various energy levels and orbitals underpins its chemical behavior. The electron placement is dictated by several rules, including the Aufbau principle, which states that electrons fill orbitals starting from the lowest available energy level.
Step-by-Step Process of Configuration
To write the electron configuration for carbon, we follow these steps:
- Start filling the lowest energy orbital, which is 1s, with electrons. The 1s orbital can hold a maximum of 2 electrons.
- Next, the 2s orbital, which can also hold 2 electrons, is filled.
- The remaining 2 electrons occupy the 2p orbital. Hence, the full electron configuration for carbon is 1s2 2s2 2p2.
Full and Condensed Notation
The full notation for carbon’s electron configuration is 1s2 2s2 2p2. In condensed notation, using the noble gas preceding carbon (helium, [He]) simplifies the expression to [He] 2s2 2p2. This shorthand is widely used for larger elements to easily indicate electron configurations without detailing every electron level.
Detailed Breakdown of Carbon’s Electron Configuration
Understanding 1s, 2s, and 2p Orbitals
The 1s, 2s, and 2p orbitals each have distinct characteristics:
- 1s Orbital: Spherical in shape and holds a maximum of 2 electrons.
- 2s Orbital: Also spherical, it can hold 2 electrons as well, existing at a higher energy level than 1s.
- 2p Orbitals: These are dumbbell-shaped and consist of three degenerative orbitals (2px, 2py, 2pz), each capable of holding 2 electrons, giving a total of 6 electrons across all three orbitals.
Role of Pauli Exclusion Principle
The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. As a consequence, each orbital can hold a maximum of two electrons with opposite spins. This principle ensures that for carbon’s 2p electrons, which total four electrons in two orbitals, they occupy different spatial distribution within their respective orbitals while maintaining opposite spins.
Hund’s Rule in Carbon’s Electron Distribution
Hund’s Rule states that electrons will fill degenerate orbitals singly first before pairing up in any of the orbitals. For carbon, this means that the two electrons in the 2p orbital will occupy two of the three available 2p orbitals before pairing in one of them. Thus, the distribution in the 2p orbital would be represented as 2px1 2py1 2pz0, rather than putting both electrons in a single orbital first.
Applications of Carbon’s Electron Configuration
Impact on Chemical Properties
The electron configuration of carbon has profound implications for its chemical properties. For instance, the four valence electrons allow carbon to form stable covalent bonds with other atoms or itself, leading to a vast array of organic molecules. Furthermore, carbon’s ability to form \(sp^3\), \(sp^2\), and \(sp\) hybridizations impacts molecular geometry, bond angles, and the types of hydrocarbons formed.
Electronegativity and Bonding Behavior
Carbon is relatively electronegative compared to metals, which means it has a tendency to attract electrons during bond formation. Its electronegativity, measured on the Pauling scale, is about 2.5. This moderate electronegativity allows carbon to engage effectively in both ionic and covalent bonding, further influencing its role in organic chemistry where it frequently bonds with hydrogen, oxygen, and nitrogen.
Understanding Hybridization in Carbon Compounds
Hybridization is a concept that describes the mixing of atomic orbitals to form new hybrid orbitals, accommodating the bonding requirements of carbon. The principal types include:
- sp3 Hybridization: In this state, one s orbital combines with three p orbitals, leading to four equivalent sp3 orbitals, as seen in methane (CH4).
- sp2 Hybridization: Here, one s orbital combines with two p orbitals, resulting in three sp2 orbitals. This hybridization characterizes molecules like ethene (C2H4).
- sp Hybridization: Involving one s and one p orbital, leading to two linear sp orbitals. Acetylene (C2H2) exemplifies this type.
Advanced Concepts and Future Perspectives
Trends in Periodic Table and Electron Configuration
As you move across a period in the periodic table from left to right, electron configurations dictate the increasing nuclear charge and the shape of the periodic table itself. Elements follow periodic trends, such as increase in ionization energy and electronegativity, profoundly affecting compound formation and chemical behavior.
Application in Molecular Orbital Theory
Molecular Orbital Theory expands the ideas of electron configuration by describing how atomic orbitals combine to form molecular orbitals that are spread over multiple atoms. This principle helps explain the properties of carbon compounds, suggesting that different hybridizations result in a variety of bonding scenarios that impact molecular properties, such as stability and reactivity.
Research Trends in Carbon Chemistry
The study of carbon’s electron configuration remains a dynamic field, with ongoing research focused on understanding carbon’s role in nanotechnology, organic semiconductors, and sustainable materials. The implications of carbon’s configurations are far-reaching, influencing not only chemistry but also materials science, energy production, and biochemistry.