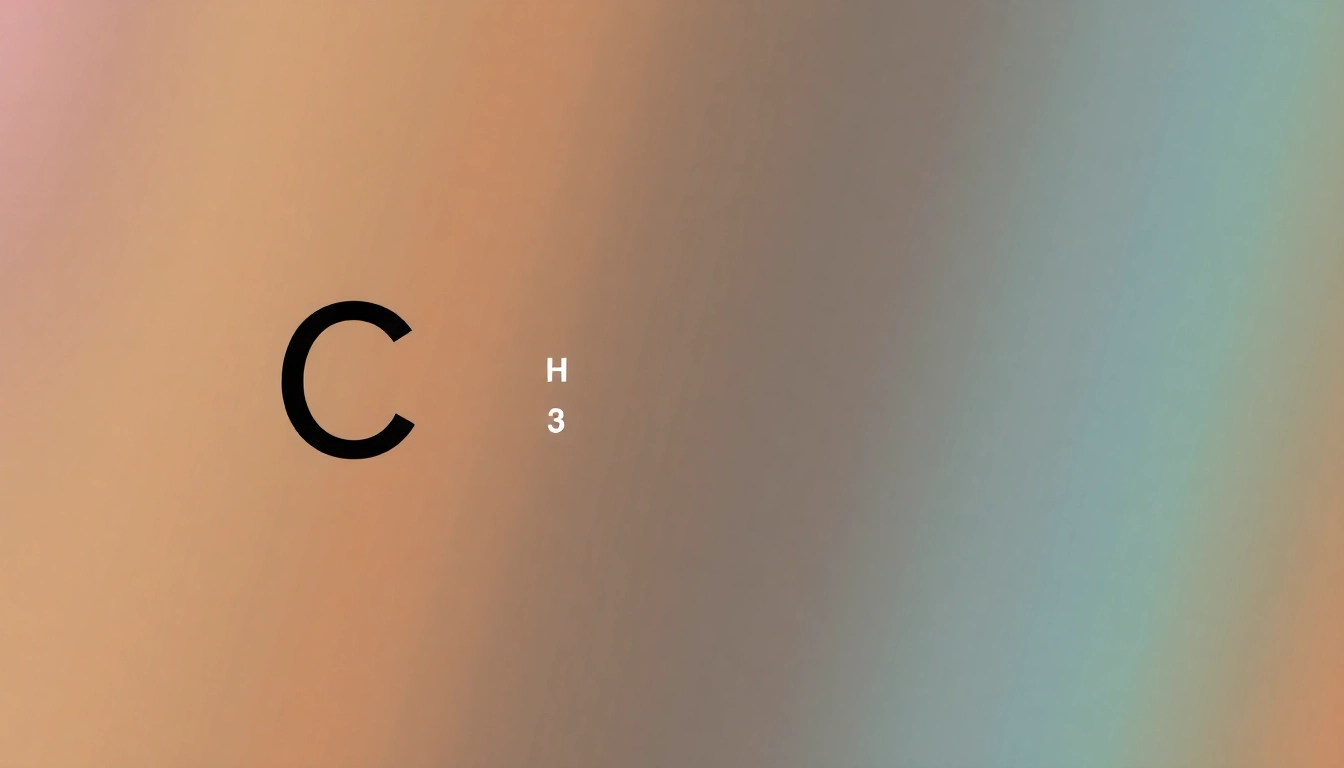Introduction to Electron Configuration
Electron configuration is a fundamental concept in chemistry that describes the distribution of electrons in an atom’s orbitals. Understanding this concept is essential for anyone seeking to deepen their knowledge of chemistry, as it lays the foundation for understanding atomic behavior, chemical reactivity, and bonding. In this article, we’ll explore c electron config, specifically focusing on the electron configuration of carbon, a pivotal element in organic chemistry and life itself.
What Is Electron Configuration?
Electron configuration refers to the arrangement of electrons in an atom. These arrangements are conventionally expressed using a set of notation that details how many electrons occupy the various orbitals (s, p, d, f) across different energy levels. Each electron configuration is specific to an element and reflects its atomic structure and properties.
Importance of Understanding c Electron Config
Carbon’s electron configuration is particularly crucial because carbon is the backbone of organic molecules. With an atomic number of 6, carbon plays a foundational role in biological chemistry. By understanding its electron configuration—1s² 2s² 2p²—students and professionals alike can grasp the implications this has on carbon’s ability to form bonds, its reactivity, and its structural versatility in various compounds.
Basic Terminology Related to Electron Configurations
To tackle electron configuration effectively, it’s helpful to understand some basic terms:
- Subshells: The specific orbitals where electrons reside, denoted as s, p, d, and f.
- Valence Electrons: The outermost electrons that play a key role in chemical bonding.
- Orbital Diagrams: Visual representations that illustrate how electrons are distributed among the orbitals within the subshells.
- Quantum Numbers: A set of four numbers that describe the unique state of an electron in an atom, including its energy and angular momentum.
The Electron Configuration of Carbon
Carbon’s electron configuration provides insight into its chemical behavior. To understand how carbon interacts with other elements, we need to break down its specific configuration.
Full Electron Configuration for Carbon (C)
The full electron configuration of carbon can be written as:
1s² 2s² 2p²
This notation indicates that carbon has two electrons in the 1s subshell, two electrons in the 2s subshell, and two electrons in the 2p subshell. Together, these account for the six electrons present in a neutral carbon atom corresponding to its atomic number.
Abbreviated Form of c Electron Config
To simplify the representation of electron configurations, chemists often use the abbreviated or condensed form. For carbon, this can also be expressed as:
[He] 2s² 2p²
In this case, “[He]” refers to helium, representing the filled inner electron shell (1s²) and allowing the chemist to focus on the outer electrons that primarily determine carbon’s chemical reactivity.
Understanding Orbital Diagrams of Carbon
Orbital diagrams provide a visual representation of how electrons are distributed across various orbitals in an atom. For carbon, the orbital diagram would appear as follows:
1s: ↑↓ (2 electrons in the s orbital)
2s: ↑↓ (2 electrons in the s orbital)
2p: ↑ ↑ (2 electrons in the p orbital, with each p orbital being half-filled to minimize repulsion)
This distribution influences carbon’s ability to bond, particularly its tetravalency, allowing it to form four covalent bonds with other elements or molecules.
Electron Configuration and Chemical Properties
The electron configuration of carbon is not just a theoretical exercise; it has real implications for the element’s chemical properties and potential reactivity. By examining these factors, we can better appreciate the nature of carbon and its applications in chemistry and biology.
How c Electron Config Influences Reactivity
The way electrons are arranged within carbon atom’s subshells directly influences its reactivity. The presence of four valence electrons allows carbon to form stable covalent bonds with a variety of elements, including hydrogen, oxygen, nitrogen, and other carbon atoms. This characteristic reactivity is a foundational principle for organic molecules, which often contain long chains and complex ring structures.
Carbon’s Role in Organic Chemistry
Carbon’s ability to bond with itself and other elements is central to the vast diversity of organic compounds. The configurations of electrons allow carbon to form single, double, and even triple bonds, contributing to an incredible array of molecular structures that can include hydrocarbons, alcohols, acids, and more. This versatility is a fundamental reason why carbon is often referred to as the building block of life.
Relations to Other Elements Based on Electron Config
In the context of the periodic table, carbon’s configuration enables interaction with neighboring elements. Understanding how carbon’s electron configuration changes relative to other elements can reveal patterns in bonding and reactivity. For example, comparing carbon’s electron configuration to that of silicon (Si), which has an electron configuration of 1s² 2s² 2p² 3s² 3p², shows us how similar group elements can exhibit both similar and distinct chemical behaviors based on their respective electron arrangements.
Common Misconceptions about Electron Configurations
Despite its significance, many misconceptions surround electron configurations. By addressing some of these common myths, we can enhance the understanding of this essential concept.
Debunking Myths about c Electron Config
A prevalent misconception is that all electrons in an atom are in a fixed position around the nucleus. In truth, electrons inhabit orbitals that represent probabilities rather than fixed paths, and their distribution is influenced by both energy levels and quantum mechanics.
Accurate vs. Simplified Representations
Another area of confusion is the difference between accurate full electron configurations and simplified forms. While abbreviating configurations provides clarity and ease of understanding, this can sometimes lead to misunderstandings about the number and arrangement of valence electrons. It’s vital to recognize that simplified notations do not lose the nuances important for understanding chemical behavior.
Understanding Hybridization and Its Impact
Hybridization, a theoretical concept used in chemistry, describes the mixing of atomic orbitals to create new hybrid orbitals tailored for bonding. In the case of carbon, hybridization results in the formation of four sp³ hybrid orbitals for tetrahedral bonding. This concept often confuses students, particularly when distinguishing between standard electron configurations and hypothetical bonding scenarios.
Advanced Concepts in Electron Configuration
Electron configuration is not merely an introductory topic; it has implications that extend into advanced chemistry principles and applications in numerous fields. Understanding these higher concepts can illuminate topics in quantum chemistry, molecular biology, and materials science.
Impact of c Electron Config on Molecular Geometry
The arrangement of electrons in carbon directly influences the molecular geometry of carbon-containing compounds. The tetrahedral shape of methane (CH₄) arises from the sp³ hybridization of carbon, where the four bonds arrange themselves in three-dimensional space to minimize electron-electron repulsion, resulting in a stable molecular structure. Similarly, the angular shape of water (H₂O), where oxygen is bonded to hydrogen atoms, is also a result of electron configuration and repulsion theory.
Quantum Mechanics and c Electron Config
Quantum mechanics plays a crucial role in understanding electron configurations. Electrons exhibit wave-particle duality, and their behavior can be described by quantum numbers. These principles dictate the configurations and energy states of electrons in an atom, anchoring the framework of modern chemistry in deeply interconnected physical theory.
Applications of Electron Configuration in Modern Chemistry
Today, electron configuration has applications across various fields of chemistry and materials science. For example, understanding electron arrangements can lead to the synthesis of new materials, develop catalysts for chemical reactions, and inform the design of pharmaceuticals based on how drugs interact with biological molecules. Additionally, the principles gleaned from electron configurations are instrumental in fields such as electronics and nanotechnology.