Introduction to Carbon and Its Electron Configuration
Carbon is one of the most fundamental elements in the universe, primarily known for its role in organic chemistry and the backbone of life as we know it. Understanding the carbon full electron configuration is crucial for students, chemists, and researchers alike. The arrangement of electrons in carbon affects its chemical behavior, bonding capabilities, and overall characteristics. This article delves into the intricate details of carbon’s electron configuration, its implications, and how to properly interpret and utilize this information in various fields of chemistry and materials science.
What Is Carbon?
Carbon, designated by the symbol C, is the sixth element in the periodic table with an atomic number of 6. It is a non-metal and is known for its ability to form various allotropes including graphite, diamond, and amorphous carbon, which exhibit vastly different physical properties. Carbon is an integral part of organic compounds, which are the building blocks of life, making its study essential. It is the sixth most abundant element in the universe and is the basis for the complex biomolecules that compose living organisms.
The Importance of Electron Configuration
Electron configuration refers to the distribution of electrons among the orbitals of an atom. This distribution governs how atoms bond and interact with one another, making it a cornerstone concept in chemistry. For carbon, understanding its electron configuration enables chemists to predict how carbon will react with other elements and compounds, the types of bonds it will form, and its role in various chemical reactions. Electron configuration is not only vital for theoretical models but also has practical implications in fields such as materials science, biochemistry, and nanotechnology.
Basic Concepts: Electrons and Orbitals
Electrons are subatomic particles with a negative charge, and they occupy various energy levels or orbitals surrounding an atom’s nucleus. The simplest model involves a series of orbitals arranged by energy level: s, p, d, and f orbitals. Each type has a specific shape and can hold a varying number of electrons. For carbon, the distribution of its six electrons across these orbitals determines its chemical properties. Understanding how electrons occupy these orbitals is fundamental to grasping carbon’s reactivity and the types of molecules it can form.
Full Electron Configuration of Carbon
The Ground State Configuration Explained
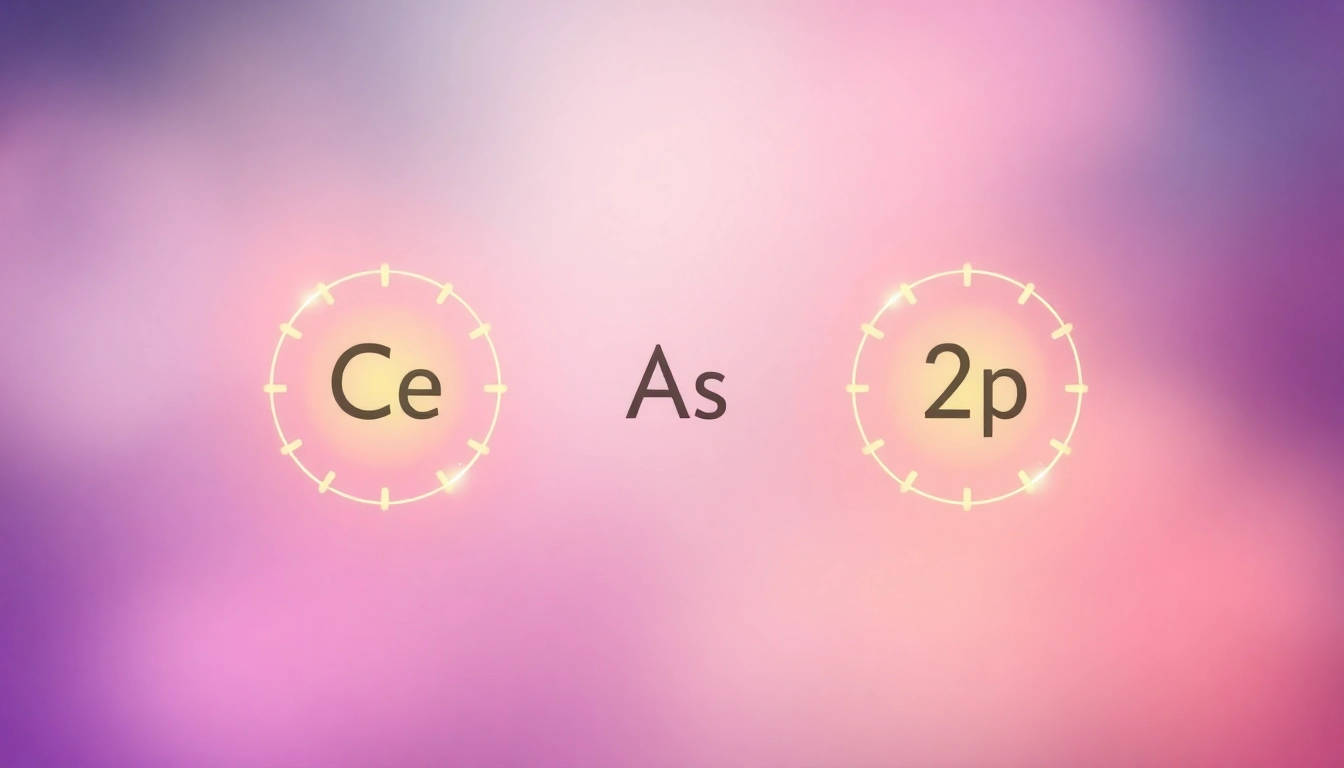
The ground state electron configuration of carbon is represented as 1s² 2s² 2p². This notation indicates that carbon has six electrons: two in the 1s orbital, two in the 2s orbital, and two in the 2p orbitals. The notation follows the Aufbau principle, which states that electrons fill orbitals starting from the lowest energy level. The 1s orbital is filled first, followed by 2s and then 2p, which can accommodate up to six electrons (three orbitals, each holding two electrons). This electron arrangement influences carbon’s tetravalent nature, allowing it to form four covalent bonds with other atoms.
Notation and Graphical Representation
Understanding carbon’s electron configuration can be further enhanced with graphical representations. The electron configuration can be visualized using orbital diagrams or electron filling charts. In these diagrams, each orbital is represented as a box with arrows indicating the presence of electrons. For carbon, the diagram shows the 1s and 2s orbitals each containing two arrows (electrons) and the 2p orbitals filled with two arrows to indicate the occupancy of the p orbitals. Diagrams provide clarity on the arrangement of electrons and can help in visualizing how electron configuration leads to bonding behaviors.
Comparison with Other Elements
When compared to other elements, carbon’s electron configuration reveals its unique chemical properties. Elements in the same group of the periodic table exhibit similar chemical behaviors due to their similar valence electron configurations. For example, silicon, which has an electron configuration of [Ne] 3s² 3p², shares similar properties with carbon regarding bonding and reactivity. However, elements further away from carbon in the periodic table, like helium (1s²) or oxygen (1s² 2s² 2p⁴), exhibit different chemical behaviors due to their differing electron configurations. Such comparisons underscore the significance of electron configurations in determining an element’s chemical identity and reactivity patterns.
How to Write Electron Configurations
Step-by-Step Process
Writing electron configurations is a systematic process that can be broken down into manageable steps:
- Identify the atomic number: For carbon, this is 6, indicating it has six electrons.
- Follow the Aufbau principle: Fill the lowest energy orbitals first (1s, followed by 2s, then 2p).
- Use Hund’s rule: In cases where multiple orbitals of the same energy level are available, one must place one electron in each orbital before pairing them.
- Write the configuration: Represent the filled orbitals, noting how many electrons are in each. For carbon: 1s² 2s² 2p².
This method not only leads to the correct electron configuration but also helps in understanding how electrons are distributed across various orbitals, ultimately affecting molecular formation.
Common Mistakes to Avoid
When writing electron configurations, it’s common to make some errors. Here are a few pitfalls to avoid:
- Skipping orbitals: Ensure you follow the correct order of filling orbitals based on energy levels.
- Incorrectly pairing electrons: Remember to fill single orbitals first according to Hund’s rule before pairing them in the same orbital.
- Neglecting the subshell filling order: For elements beyond iron, the 3d orbital fills before the 4s in energy considerations, which can lead to errors if not properly accounted for.
Learning to avoid these common mistakes can significantly enhance your proficiency in writing and understanding electron configurations across various elements.
Tools and Resources for Practice
Various resources are available for mastering electron configurations:
- Online simulations: Websites and applications offer interactive models to visualize electron configurations.
- Study guides: Numerous textbooks and online courses provide structured lessons on orbital diagrams and electron configurations.
- Practice problems: Engage with problems that require you to write electron configurations for various elements, which reinforces learning through application.
Utilizing these resources can enhance your understanding and ability to accurately determine the electron configurations of various elements.
Applications of Electron Configuration in Chemistry
Understanding Chemical Properties
The electron configuration of an atom is deeply tied to its chemical properties. The distribution of electrons determines how an atom interacts with others, forming bonds. For instance, carbon’s four valence electrons allow it to form stable covalent bonds with a variety of other elements, leading to the diversity of organic compounds. Understanding electron configurations grants insights into the reactivity and stability of different substances, impacting fields ranging from medicinal chemistry to materials science.
Electron Configuration and Reactivity
The reactivity of an element is significantly influenced by its electron configuration. Elements can lose, gain, or share electrons based on their configurations to achieve a stable electron arrangement, often resembling that of noble gases. For carbon, its tetravalent nature facilitates the formation of diverse organic compounds, including hydrocarbons, alcohols, and amino acids. These interactions are essential for understanding biochemical pathways and the functionality of various chemical alloys and compounds.
Trends Across the Periodic Table
Electron configuration provides a foundational perspective on trends observed in the periodic table. The placement in the periodic table reflects the number of electrons in the outermost shell, influencing an element’s properties, including ionization energy and electronegativity. As you move across a period, atomic number increases, and electron configuration progresses from filling one subshell to the next, leading to observable trends in element behavior—carbon serves as a pivotal element illustrating how foundational principles emerge from configurations.
Challenges and Advanced Concepts
Excited States and Ions
An electron can absorb energy and move to a higher energy orbital, resulting in an excited state. In the case of carbon, its ground state is 1s² 2s² 2p², but it can temporarily transition to configurations such as 1s² 2s² 2p¹ 3s¹ under certain conditions when additional energy is supplied. Moreover, ions exhibit different electron configurations than neutral atoms, characterized by the loss or gain of electrons to achieve stability. Understanding excited states and the ionization process enhances one’s ability to comprehend molecular behavior and reactions in dynamic systems.
Complex Configurations in Transition Metals
While this article focuses primarily on carbon, it’s essential to recognize that transition metals exhibit more complex electron configurations, often involving the d and f orbitals. These configurations can lead to unique properties such as paramagnetism and anomalous oxidation states. The study of these elements requires a deep understanding of quantum mechanics and electron interactions, expanding the applications of electron configurations into advanced fields like catalysis, material science, and condensed matter physics.
Future Trends in Research
As research in chemistry continues to evolve, the role of electron configuration will be crucial in the development of new materials, pharmaceuticals, and nanotechnology applications. Innovations in computational chemistry and quantum simulations are allowing scientists to predict and manipulate electron configurations to achieve desired properties in new compounds, facilitating breakthroughs in energy storage and conversion technologies. The exploration of unusual electron configurations could lead to the discovery of novel materials with unprecedented capabilities, marking an exciting frontier in chemical research.