Introduction to Carbon and Its Electronic Structure
Atomic Number and Basic Properties
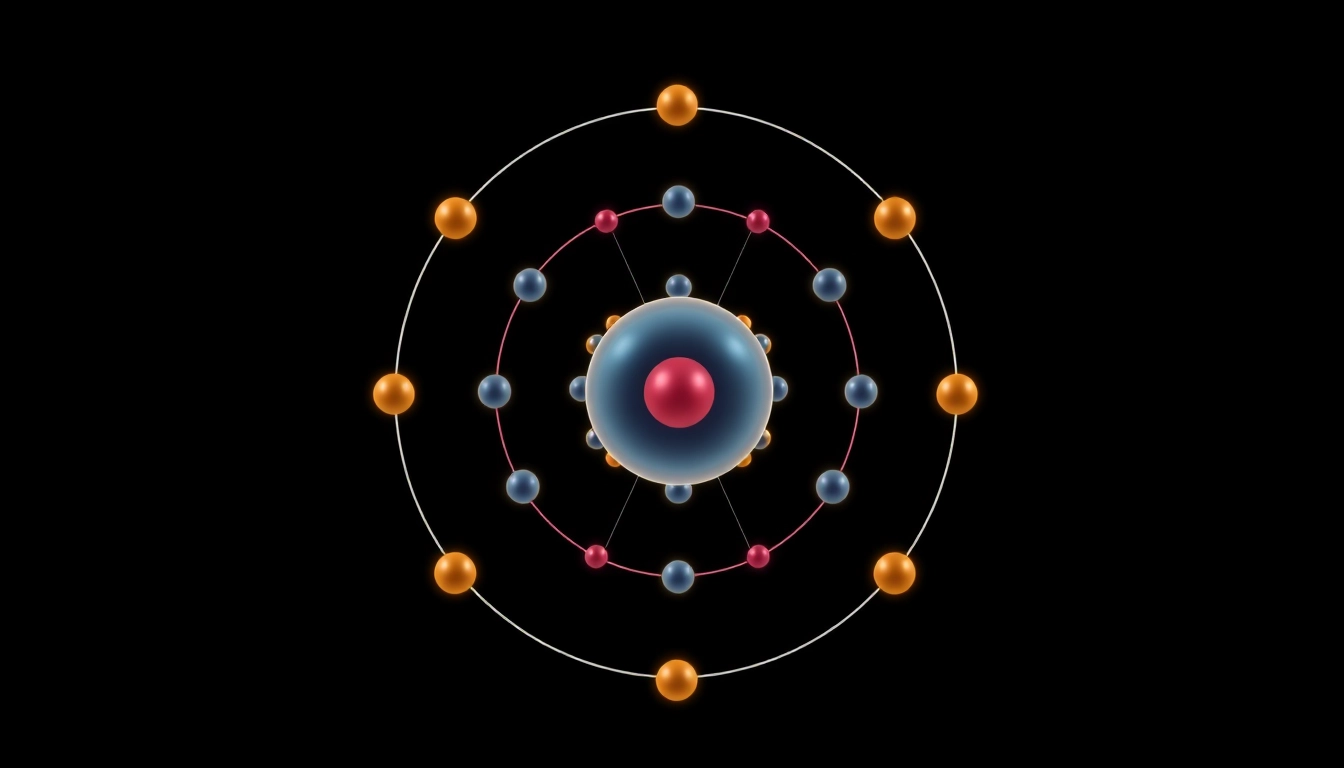
Carbon, represented by the symbol C, is the sixth element in the periodic table, with an atomic number of 6. This means that it contains 6 protons and typically 6 electrons in its neutral state. Carbon is unique in that it is the foundational element for all known life, forming the backbone of numerous organic molecules. Its ability to bond with a wide variety of other elements helps create complex structures, including proteins, nucleic acids, and hydrocarbons.
Understanding Electron Configurations
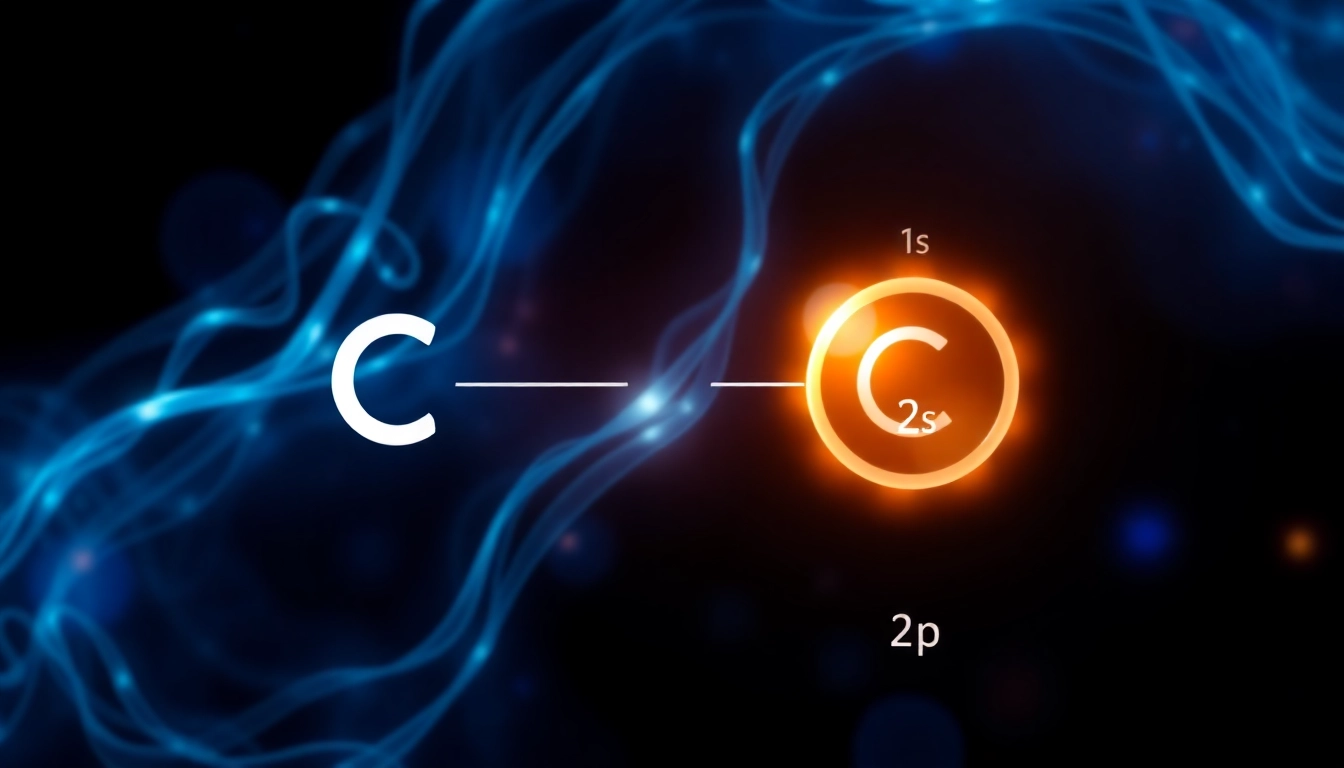
The electron configuration of an atom describes the distribution of its electrons across various energy levels and orbitals. For carbon, the electron configuration is a vital piece of information, as it lays the groundwork for understanding its chemical behavior. The standard representation of carbon’s electron configuration is as follows: carbon electronic configuration often presented in the notation of 1s², 2s², and 2p², denoting the occupation of electrons in each respective subshell.
Importance of Electron Configuration in Chemistry
Electron configurations are crucial for predicting how different elements will interact and bond with one another. The arrangement of electrons in an atom influences its ionization energy, electronegativity, and atomic radius. As a result, understanding the electron configuration of carbon helps chemists predict the nature of its chemical reactions, its multiple allotropes (such as diamond and graphite), and its various bonding capabilities that are central to organic chemistry.
Basic Electronic Configuration of Carbon
Ground State of Carbon
The ground state electron configuration of carbon is represented as 1s² 2s² 2p². This indicates that in its lowest energy state, carbon has two electrons in the 1s orbital, two in the 2s orbital, and two in the 2p orbital. Notably, the two p electrons can occupy three separate p orbitals (2p_x, 2p_y, and 2p_z), which play a critical role in the atom’s bonding behavior.
Orbital Filling Order and Rules
Carbon’s electron configuration is governed by several principles of quantum chemistry. The Aufbau principle states that electrons fill orbitals starting from the lowest energy level moving to higher levels. Pauli’s exclusion principle indicates that no two electrons can have the same set of quantum numbers, which reinforces the variety of arrangements seen in orbital diagrams. Hund’s rule states that electrons will fill degenerate orbitals singly before pairing up. This leads carbon to be capable of forming four covalent bonds, using its four valence electrons effectively.
Notation for Carbon’s Electron Configuration
The notation for carbon’s electron configuration can be condensed to raise clarity and simplicity. Instead of writing out full configurations, we often use noble gas shorthand. For carbon, this would place the configuration in relation to the noble gas helium, resulting in the abbreviation: [He] 2s² 2p². This notation emphasizes the most significant outer-shell electrons while minimizing the complexity of representing inner shell electrons.
Advanced Concepts in Carbon’s Electron Configuration
Exceptions to the Aufbau Principle
While the Aufbau principle provides a foundational guideline for determining electron configurations, there are notable exceptions, especially in transition metals. However, in the case of carbon, it adheres closely to this principle due to its stability and relatively low atomic number, allowing for a straightforward electron arrangement.
Impact of Electron Configuration on Reactivity
Carbon’s electron configuration directly influences its reactivity and the types of chemical bonds it can form. The four unpaired electrons in carbon’s outer orbitals enable it to form four covalent bonds via hybridization. This hybridization allows diverse molecular geometries, such as tetrahedral (as in methane) and planar (as in ethylene), which drastically impact molecular properties and functional characteristics.
Comparison with Related Elements
When examining the group of elements related to carbon in the periodic table, particularly silicon, germanium, and others in group 14, we observe similarities but also distinctions in their electron configurations. For instance, silicon has an electron configuration of [Ne] 3s² 3p². As we move down this group, the increase in atomic size and electron shielding affects bonding properties and reactivity, which can inform predictions in various chemical applications.
Visual Representation of Carbon’s Electron Configuration
Orbital Diagrams for Clarity
Orbital diagrams visually represent how electrons are distributed among orbitals in an atom. For carbon, the 1s and 2s orbitals are fully populated, while the 2p orbitals can be represented with arrows indicating the unpaired electrons. This visualization vividly illustrates the underlying structure of carbon and highlights its bonding potential by showing how these electrons can interact during chemical reactions.
Understanding Hybridization Concepts
Carbon can undergo hybridization, which is the mixing of atomic orbitals to form new hybrid orbitals suitable for pairing electrons to form chemical bonds. The most common types of hybridization for carbon are sp³, sp², and sp, leading to tetrahedral, trigonal planar, and linear geometries, respectively. Understanding these hybridizations is paramount in explaining the vast array of organic compounds, as well as variations in hybridization that contribute to the unique physical properties of materials.
Application in Organic Chemistry
In organic chemistry, carbon’s electron configuration is central to the study of the structure, properties, and reactions of organic molecules. The ability of carbon to form stable bonds with itself and other elements leads to a vast diversity of organic compounds. The principles of electron configuration and hybridization lay the groundwork for understanding functional groups, isomerism, and reaction mechanisms fundamental to organic synthesis.
Conclusion and Summary of Key Points
Recap of Carbon’s Electron Configuration
In summary, the *electron configuration of carbon* is essential to grasp its place in the periodic table and its chemical behavior. It dictates how carbon interacts with other elements, ultimately enabling the complex chemistry that supports life. Understanding the ground state configuration of 1s² 2s² 2p² is vital for predicting carbon’s reactivity and bonding.
Importance in Molecular Formation
Carbon’s capacity to bond covalently with a multitude of elements hinges upon its unique electron configuration. This allows for the formation of diverse molecular structures, which are foundational to biochemistry and materials science. The interplay of carbon’s electron arrangement with hybridization concepts explains the rich chemistry of organic molecules and their transformations.
Discussion on Future Research Directions
The study of carbon’s electron configuration remains a dynamic field, intersecting with advances in materials science, nanotechnology, and synthetic biology. Future research may further unravel the intricacies of carbon bonding in novel materials and upcycling technologies while exploring carbon-based life’s crucial role in sustainability and environmental science.