Introduction to Carbon and Its Electron Configuration
Carbon is a fundamental element in the universe, known as the building block of life. It has an atomic number of 6, meaning it contains six protons, and consequently, six electrons. Understanding the carbon electron distribution is crucial, as it lays the foundation for comprehending the behavior of carbon in various chemical reactions and its role in chemistry at large. This article aims to provide a comprehensive overview of carbon’s electron distribution, including its significance, the basic principles governing electron arrangement, and the implications of carbon’s configuration on its chemical properties.
What is Carbon?
Carbon is a non-metallic element essential for all known life forms. It exists in various allotropes, including graphite, diamond, and fullerenes, each showcasing different physical properties due to the arrangement of carbon atoms and their electron configurations. The knowledge of carbon is not limited to chemistry but extends to fields such as biology, environmental science, and materials science due to its ubiquity in organic molecules, the backbone of life.
Significance of Electron Configuration
The electron configuration of an atom provides critical insights into its chemical properties. It describes how electrons are distributed in an atom’s orbitals, affecting the atom’s reactivity, ionization energy, electronegativity, and bonding behaviors. In carbon’s case, understanding its electron distribution helps explain its ability to form stable covalent bonds with other elements, leading to the vast diversity of organic compounds.
Basic Principles of Electron Distribution
To grasp electron distribution, we must familiarize ourselves with several foundational principles of quantum mechanics and atomic structure:
- Aufbau Principle: Electrons occupy the lowest energy orbitals first before moving to higher ones.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of quantum numbers, meaning each orbital can hold a maximum of two electrons with opposite spins.
- Hund’s Rule: Every orbital in a subshell is singly occupied before any orbital is doubly occupied, which minimizes electron-electron repulsions and stabilizes the atom.
Detailed Breakdown of Carbon Electron Distribution
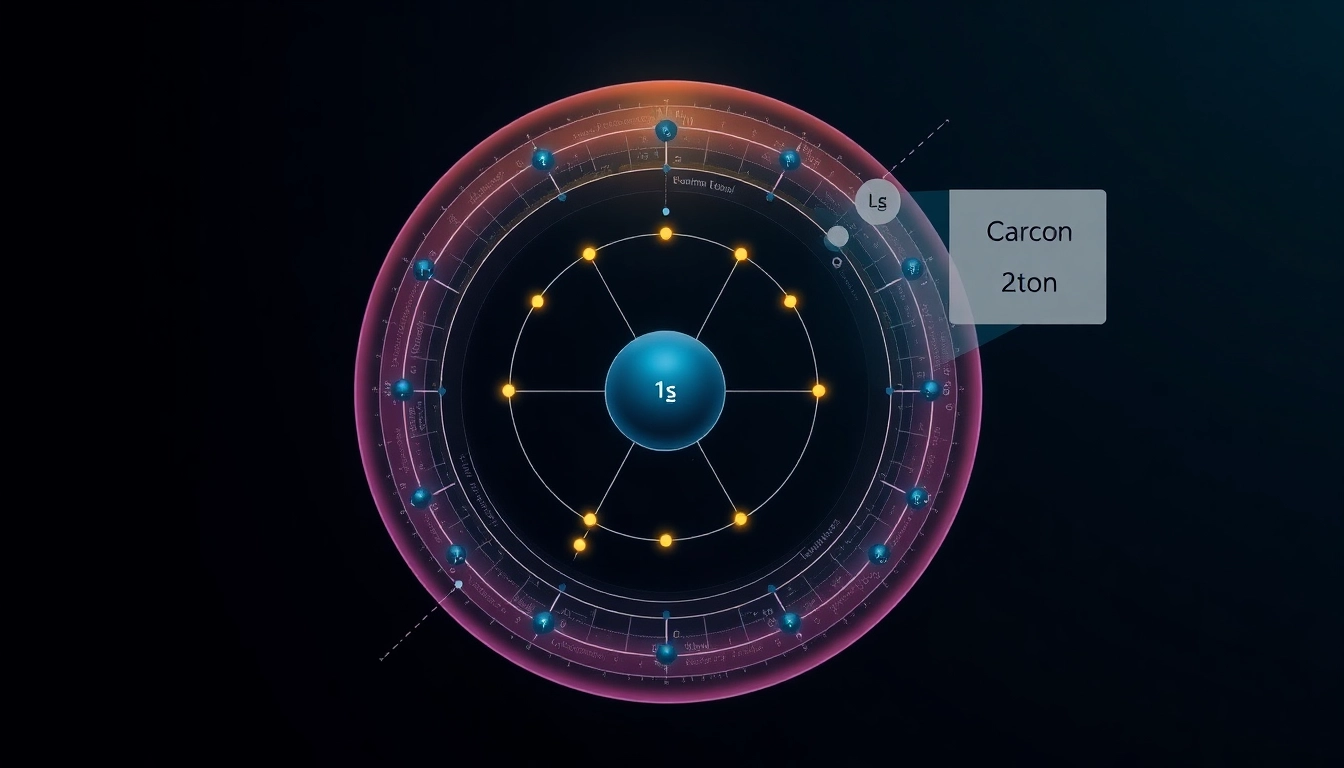
1s Orbital: The Foundation of Carbon
In carbon, the electron distribution begins with the 1s orbital, which can hold a maximum of two electrons. Both of carbon’s first two electrons are placed in this orbital, providing the foundational electron distribution. The full notation for the 1s orbital is written as 1s2, indicating two electrons occupy this space. This arrangement establishes a stable initial state for the atom, making it less reactive until additional electrons are added to higher energy levels.
2s and 2p Orbitals: Expanding the Structure
After filling the 1s orbital, we move to the 2s orbital, which can also hold up to two electrons. Hence, the next two electrons in carbon will occupy this orbital (2s2). The remaining two electrons are distributed in the 2p orbitals, which are designated as 2px, 2py, and 2pz. As per Hund’s rule, one of the last two electrons goes into the 2px orbital, and the other occupies the 2py orbital (2p2). Thus, the complete electron configuration of carbon is 1s2 2s2 2p2.
Interpreting Electron Distribution Diagrams
Electron distribution diagrams visually represent how electrons are arranged within an atom. For carbon, a common way to illustrate this is through orbital diagrams, where arrows represent electrons. Each orbital is depicted as a box or line, and the direction of each arrow indicates the spin of the electron. By analyzing these diagrams, one can gain a clearer understanding of an atom’s reactivity and its potential to form bonds with other elements.
The Role of Carbon in Chemistry
Chemical Properties Influenced by Electron Arrangement
Carbon’s unique electronic structure allows it to form four covalent bonds with other atoms, including other carbon atoms. This tetravalency and the electron configuration of 1s2 2s2 2p2 enable carbon to engage in a variety of chemical reactions, resulting in a vast array of organic compounds. Furthermore, the balance of occupied and unoccupied orbitals influences chemical properties like electronegativity and ionization energy, making carbon relatively stable under standard conditions.
Carbon’s Valence Electrons and Bonding
The four valence electrons in carbon’s outer shell play a crucial role in its bonding capabilities. Carbon can achieve a full outer shell by sharing electrons, leading to single, double, or triple bonds with various elements, including hydrogen, oxygen, nitrogen, and other carbon atoms. This ability to bond in multiple ways allows for complex molecules such as sugars, proteins, and DNA, showcasing carbon’s versatility in forming life-sustaining compounds.
Applications in Organic Chemistry
Organic chemistry, the study of carbon-containing compounds, heavily relies on the principles of carbon’s electron distribution. Reactions, including substitution, addition, and elimination processes, are all influenced by how carbon atoms share and distribute their electrons. Knowledge of electron distribution patterns can also assist chemists in predicting the reactivity of specific compounds and designing new molecules with desired properties, underlining the significance of understanding carbon’s electronic structure in both academic and industrial applications.
Common Questions about Carbon Electron Distribution
How is Carbon’s Electron Configuration Determined?
The electron configuration for carbon can be determined using quantum mechanics and the principles outlined earlier. With carbon having six electrons, the electron distribution follows a systematic filling of orbitals by considering increasing energy levels as per the Aufbau principle. Tools such as the periodic table can also aid in predicting the electron configurations of other elements based on their position.
Application of the Aufbau Principle to Carbon
The Aufbau principle dictates that electrons fill the lowest energy orbitals first, which directly applies to the electron distribution observed in carbon. In carbon’s progression from 1s to 2s and finally to the 2p orbitals, we see this principle in action. Such a systematic approach helps predict the electron configurations of similar elements within the same group on the periodic table.
Related Concepts: Carbon’s Isotopes and Ions
Beyond its electron distribution, carbon exists in different isotopic forms, characterized by varied neutron counts, while still maintaining its six electrons. Isotopes such as Carbon-12 and Carbon-14 exhibit the same chemical properties due to identical electron configurations but differ in their nuclear stability. Furthermore, carbon can lose or gain electrons to form ions, such as the carbocation (C+) or carbanion (C–), which are integral to organic reaction mechanisms.
Conclusion: The Importance of Understanding Carbon’s Electron Distribution
Summary of Key Points
Understanding carbon’s electron distribution is paramount in the realm of chemistry. With six electrons distributed as 1s2 2s2 2p2, this unique arrangement allows carbon to engage in myriad chemical bonds, forming the basis for organic compounds essential for life. The principles governing this distribution provide insights into chemical reactivity, bonding behavior, and stability of carbon-based materials.
Implications for Future Chemistry Studies
A comprehensive grasp of carbon’s electronic structure is fundamental for future studies in chemistry, particularly as we venture into new realms of synthetic chemistry and molecular biology. Researchers can apply these principles to discover novel compounds and improve methods of synthesis that can have significant implications in various scientific fields, from pharmaceuticals to renewable energy.
Resources for Further Learning
For readers seeking to deepen their understanding of carbon and its attributes, the following resources can serve as valuable tools:
- Carbon Overview by Chemistry World
- Khan Academy Chemistry Course
- PV Education’s Guide to Carbon Electron Configuration