Understanding Carbon and Its Atomic Structure
Introduction to Carbon
Carbon is an element synonymous with life as we know it, forming the backbone of organic compounds. With an atomic number of 6, carbon is strategically positioned in group IVA of the periodic table, nestled between boron and nitrogen. Its unique properties stem from its atomic structure, where it comprises 6 protons, 6 neutrons, and 6 electrons. The intricate arrangement of these electrons is pivotal in defining not only its chemical reactivity but also its role in forming the vast array of compounds essential for life. Understanding the electron arrangement for carbon unlocks a world of insights into its bonding behavior and reactivity, making it a fundamental topic in chemistry.
Importance of Electron Configuration
Electron configuration refers to the distribution of electrons in an atom’s orbitals. For carbon, the arrangement is articulated as 1s2 2s2 2p2. This notation reflects the number of electrons in each sublevel and is crucial for predicting an element’s chemical behavior. Proper knowledge of electron configurations allows chemists to ascertain how elements interact with one another, their oxidation states, and their ability to conduct electricity or form ionic and covalent bonds. Understanding carbon’s configuration lays foundational knowledge for fields ranging from organic chemistry to materials science.
Overview of Atomic Structure
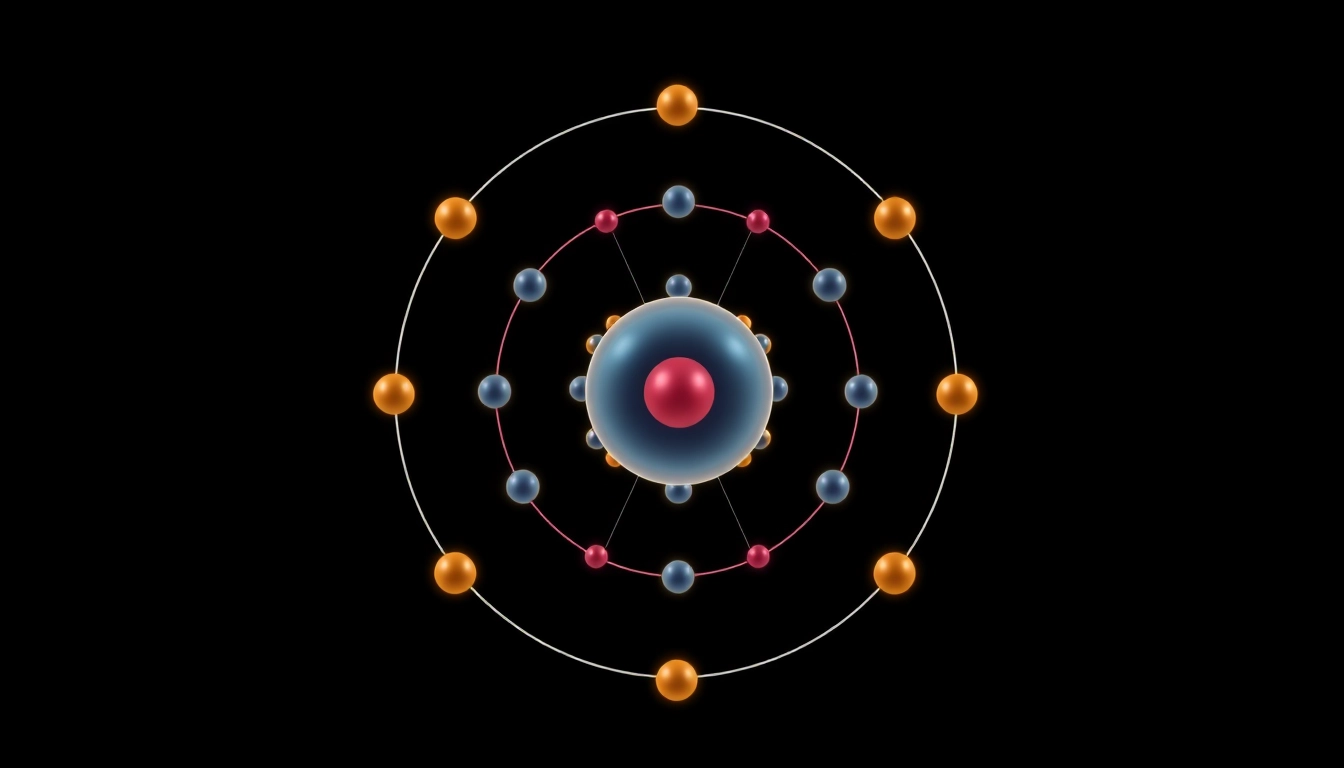
At the heart of every atom lies a nucleus comprised of protons and neutrons, surrounded by a cloud of electrons occupying specific energy levels or shells. In carbon’s case, its 6 electrons occupy the first two principal energy levels. The first shell can hold a maximum of 2 electrons, while the second can accommodate up to 8. Carbon’s electron distribution (1s2 2s2 2p2) indicates that two electrons fill the innermost shell (1s), while the ones in the second shell (2s and 2p) are responsible for forming chemical bonds. This arrangement is the key to understanding carbon’s tetravalency — its ability to form four bonds, which is central to the structure of organic molecules.
The Electron Arrangement for Carbon Explained
Basic Configuration: 1s2 2s2 2p2
The electron arrangement for carbon is succinctly summarized in the notation 1s2 2s2 2p2. This configuration indicates that carbon has:
- 2 electrons in the first shell (1s2).
- 2 electrons in the second shell’s s-orbital (2s2).
- 2 electrons in the second shell’s p-orbitals (2p2).
The filling of these orbitals adheres closely to principles governed by quantum mechanics, including the Pauli exclusion principle, Hund’s rule, and the Aufbau principle, ensuring the electrons are arranged in the lowest available energy levels before filling higher ones. This fundamental principle of electron configuration is crucial for predicting how carbon will react chemically.
Shell Levels and Electron Distribution
Carbon’s atomic structure includes two energy levels or shells. The first shell holds a maximum of two electrons, while the second shell can contain up to eight. In carbon’s arrangement, the first two electrons fill the 1s orbital, thus completing the first energy level. In the second energy level, the 2s orbital can hold a maximum of two electrons, which are filled next, followed by the 2p orbitals. The remaining four available spots in the 2p subshell are crucial for carbon’s bonding characteristics.
When considering the second shell, four electrons are present: two in the 2s orbital and two in the 2p orbitals. This spatial distribution equates to carbon’s tetravalency, allowing it to form four covalent bonds with other elements or atoms, effectively making it one of the most versatile elements in the periodic table.
Visualizing Carbon’s Electron Arrangement
Visual tools such as electron configuration diagrams and Lewis dot structures can be invaluable in comprehending carbon’s electron arrangement. An electron configuration diagram shows how electrons populate the various orbitals, while Lewis dot structures depict the valence electrons that participate in bond formation. For carbon, the Lewis structure would illustrate four valence electrons around the carbon atom, denoting its capability to engage in various bond formations, whether single, double, or even triple bonds, with different atoms.
The Role of Electron Arrangement in Chemical Properties
Bonding and Reactivity of Carbon
The electron arrangement profoundly influences carbon’s bonding capabilities. With four electrons available for bonding (two in the 2s and two in the 2p orbitals), carbon can form up to four covalent bonds with other atoms. This tetravalency is fundamental in constructing simple hydrocarbons like methane (CH4) all the way to complex biological macromolecules like proteins and nucleic acids.
Furthermore, carbon atoms can bond with themselves, leading to various structural forms, including chains, branched structures, and rings. This characteristic allows for an immense diversity of organic compounds, setting carbon apart as a cornerstone of organic chemistry.
Comparative Analysis: Carbon vs. Other Elements
Carbon’s electron arrangement differentiates it from many other elements, particularly those in its vicinity in the periodic table. Elements like silicon, which has four valence electrons as well, result in similarities in bonding characteristics but lack the same versatility and stability in complex structures as carbon. For instance, whereas silicon can form chains and tetrahedral structures, these are generally less stable and reactive than those formed by carbon.
In contrast, elements like nitrogen and oxygen, despite having electron configurations capable of multiple bonding, don’t achieve the same structural complexity as carbon due to factors like electronegativity and the inability to form stable structures that carbon efficiently accommodates. This comparative analysis accentuates the unique position of carbon in chemistry.
Implications of Electron Configuration in Organic Chemistry
The implications of carbon’s electron configuration are far-reaching in organic chemistry. From hydrocarbons (alkanes, alkenes, alkynes) to functional groups (alcohols, amines, carboxylic acids), the ability of carbon to bond in various ways directly influences the chemical properties, reactivity, and stability of organic molecules.
Furthermore, the diverse bonding capabilities of carbon allow for isomerism — the existence of compounds with the same formula but different structures — which underpins much of organic synthesis and reaction mechanisms. This complexity calls for deep understanding, making organic chemistry amidst the most fascinating branches of chemistry.
Common Misunderstandings About Electron Configuration
Clarifying the Electron Configuration Terms
Many common misconceptions arise from terminology surrounding electron configuration. One prevalent misunderstanding is confusing electron configuration with atomic mass or atomic number. The atomic number (6 for carbon) reflects the number of protons in the nucleus, while electron configuration details how those electrons are arranged among available orbitals.
Moreover, terminology like ‘octet rule,’ referring to the tendency of atoms to prefer having eight electrons in their valence shell, can lead to oversimplified interpretations when applied to carbon. Its ability to bond in complex ways challenges this notion and demonstrates that while carbon often seeks stability through acquiring an octet, it can and does form stable compounds with fewer or more than eight surrounding electrons.
Common Errors in Electron Arrangement for Carbon
Another area of confusion often includes misapprehension regarding the state of electron arrangements in ions versus neutral atoms. For example, while carbon in its neutral state has a configuration of 1s2 2s2 2p2, a carbon ion (such as the carbide ion, C4-) would have an electron arrangement accounting for additional electrons, potentially changing its chemical behavior drastically.
Separating these configurations is vital in understanding chemical reactivity and potential charge interactions in reactions and compound formations.
Factual Insights from Educational Resources
Various educational resources offer diagrams, animations, and visual representations of electron configurations for carbon that can aid in overcoming common misunderstandings. Websites like TerpConnect provide demonstrative examples of how carbon’s electrons fill orbitals, emphasizing the principles that govern these behaviors, such as the Aufbau principle and Hund’s rule.
Access to such resources can enhance comprehension and facilitate learning among students and novice chemists. Engaging with interactive tools may clarify complex concepts surrounding configuration that traditional textbook examples might obscure.
Advanced Topics in Electron Configuration
Quantum Mechanics and Electron Behavior
At the heart of understanding electron arrangements lies quantum mechanics, which governs electron behavior at the atomic and subatomic levels. Carbon’s electron distribution can be further explored through quantum mechanics, detailing the probabilistic nature of electron locations within orbitals.
The uncertainty principle implies that while we can predict where an electron is likely to be found, we cannot pinpoint its exact location at all times. This understanding of electron behavior can lead to enhanced chemical modeling and simulation techniques, offering insights into carbon’s potential reactions and bond formations in various environments.
Future Trends in Carbon Research
Looking ahead, research into carbon’s electron arrangement influences various modern scientific disciplines. The exploration of carbon nanomaterials like graphene showcases the unique properties derived from its electron configuration, opening avenues in areas like electronics and nanotechnology.
Developments in computational chemistry generate predictive models illustrating how electronic arrangements will behave under different structural modifications, offering environmentally friendly applications through carbon-based materials in energy storage, catalysis, and biomedical devices.
Applications of Understanding Electron Arrangement
Understanding carbon’s electron arrangement is not merely an academic exercise but holds practical applications in real-world scenarios. Carbon’s ability to form stable bonds and complex structures leads to its widespread use in pharmaceuticals, materials science, and even nanotechnology.
By leveraging the unique properties of carbon’s electron configuration, chemists and material scientists can design innovative drugs, sustainable materials, and advanced technologies. Understanding the fundamental principles behind electron arrangements forms the backbone of progressive research in chemistry and other allied fields.