Introduction to Electronic Configuration of Carbon
The what is the electronic configuration of carbon topic poses a significant question in the study of chemistry. As one of the foundational elements of organic chemistry, understanding carbon’s electron configuration is critical for grasping fundamental concepts in chemical bonding and molecular structure. This article delves into the intricate details of carbon’s electron configuration, breaks down its significance in various chemical contexts, and highlights common misconceptions and practical applications in both theoretical and applied chemistry.
What is Electron Configuration?
Electron configuration refers to the distribution of electrons in an atom’s orbitals. Each electron occupies a specific area, referred to as an orbital, and follows specific rules, including the Aufbau principle, Hund’s rule, and the Pauli exclusion principle. These principles guide how electrons fill atomic orbitals in a way that determines an atom’s chemical behavior.
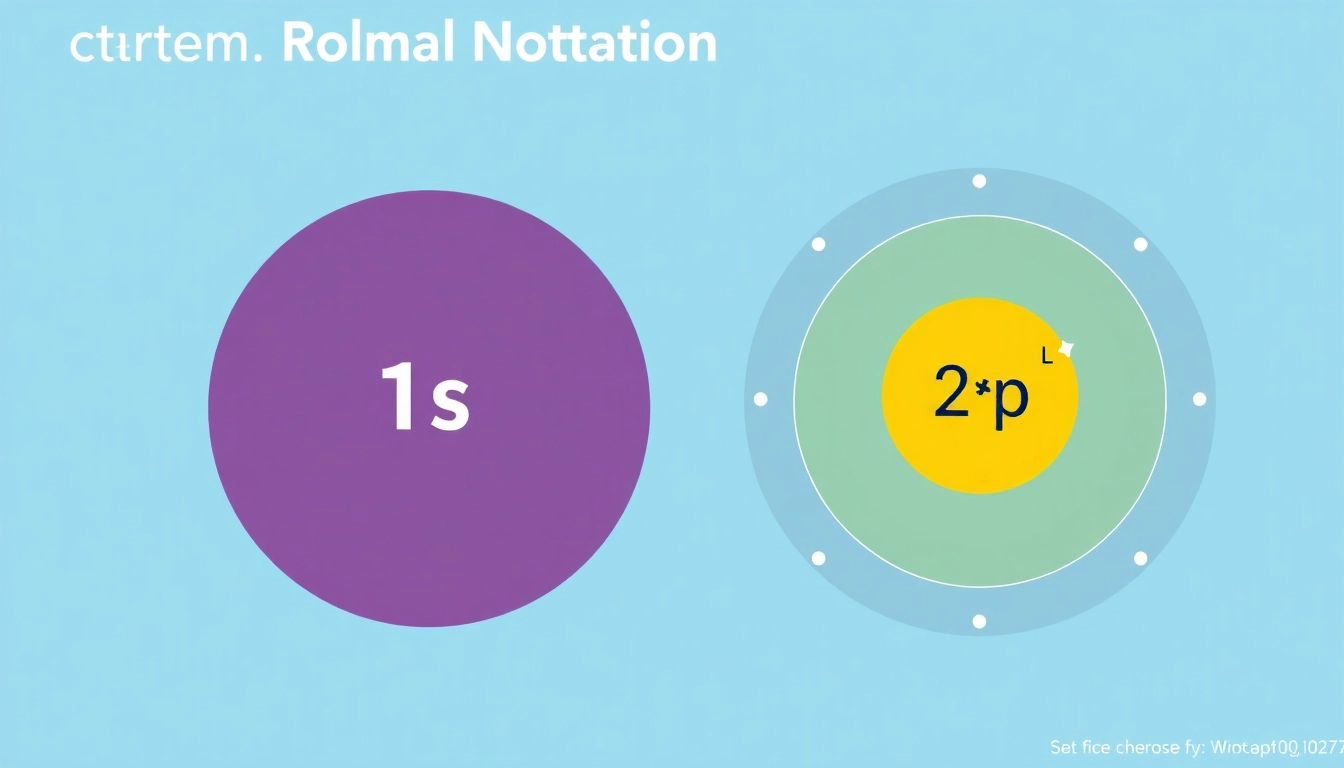
The configuration is typically expressed in a notation format that includes the principal quantum number, subshell type, and superscripts indicating the number of electrons in those subshells. For example, carbon’s electron configuration is represented as 1s² 2s² 2p².
Significance of Carbon in Chemistry
Carbon holds an exceptional place in chemistry as it is the primary building block of all known life forms. With the ability to form four covalent bonds due to its four valence electrons, carbon is instrumental in creating a variety of organic molecules, including carbohydrates, proteins, lipids, and nucleic acids. Furthermore, carbon’s unique properties allow for the formation of complex structures and molecules through various bonding patterns, making it the basis for organic chemistry.
Basic Electron Shell Structure
The electron shell model explains the arrangement of electrons in an atom. Electrons occupy energy levels or shells around the nucleus, starting from the shell closest to the nucleus outward. Each shell is divided into subshells: s, p, d, and f. Carbon, with an atomic number of 6, has a total of 6 electrons distributed across its electron shells:
- First shell: 2 electrons in the 1s subshell
- Second shell: 2 electrons in the 2s subshell and 2 electrons in the 2p subshell
The arrangement follows the order of increasing energy levels, leading to the electron configuration of carbon as 1s² 2s² 2p².
Detailed Electron Configuration of Carbon
The Notation Explained: 1s² 2s² 2p²
The notation 1s² 2s² 2p² succinctly conveys how electrons are arranged in carbon. The notation can be understood as follows:
- 1s²: Indicates that the first energy level (n=1) has two electrons in the s subshell (the maximum capacity of an s subshell is 2).
- 2s²: Signifies that the second energy level (n=2) also contains two electrons in the s subshell.
- 2p²: Shows that the second energy level has two electrons in the p subshell, which can hold a maximum of 6 electrons.
This distribution balances energy levels to maintain a stable electron arrangement. As a result, the carbon atom achieves a stable state, characterized by its ability to form diverse bonds with other elements.
Writing the Configuration Step-by-Step
To write the electron configuration for carbon, follow these steps:
- Identify the atomic number of carbon, which is 6.
- Fill the orbitals according to the Aufbau principle, starting from the lowest energy level and moving upward.
- Place 2 electrons in the 1s orbital.
- Move to the second energy level and place 2 electrons in the 2s orbital.
- Finally, place the remaining 2 electrons in the 2p orbital.
By following these steps, you can derive the electron configuration 1s² 2s² 2p² for carbon systematically.
Comparing with Other Elements
Understanding carbon’s electron configuration becomes clearer when compared to the configurations of other elements. For example:
- Hydrogen (H): 1s¹
- Helium (He): 1s²
- Boron (B): 1s² 2s² 2p¹
- Nitrogen (N): 1s² 2s² 2p³
Through these comparisons, one can observe that carbon’s configuration allows for greater versatility than hydrogen and helium, yet less than nitrogen. Carbon’s four bonding electrons enable the formation of multiple molecular structures, whereas nitrogen’s five electrons grant it the ability to form three bonds.
Common Misconceptions about Carbon’s Electron Configuration
Variations in Notation
Misunderstandings surrounding the notation of electron configurations frequently arise, particularly regarding condensed or abbreviated forms. Some may represent carbon as [He] 2s² 2p², where [He] denotes the electron configuration of helium (1s²) as a shortcut to simplify notation. This representation is valid and widely accepted in professional chemistry.
Understanding Exceptions
While carbon follows the typical rules of electron configuration, exceptions to general rules exist, particularly within transition metals where electron configurations can be influenced by factors such as electron pairing energy and effective nuclear charge. However, in the case of carbon and its group 14 counterparts, such exceptions are minimal and do not affect its core electron configuration structure.
Importance of Accurate Notation
Accurate electron configuration notation is crucial for predicting the chemical properties and reactivity of an element. Confusion with similar notations, such as pairing differences (showing 2p¹ 2p¹ vs. 2p²), can lead to misunderstandings in chemical theory and practice. For educational purposes, understanding the underlying principles that govern electron configuration helps prevent such errors.
Practical Applications of Carbon’s Electron Configuration
Role in Chemical Bonds
Carbon’s electron configuration shapes its large capacity for forming covalent bonds, cementing its role in organic chemistry. The presence of four valence electrons leads to various types of bonding, including:
- Single Bonds: Formed when two atoms share one pair of electrons (e.g., methane, CH₄).
- Double Bonds: Created when two pairs of electrons are shared between atoms (e.g., ethene, C₂H₄).
- Triple Bonds: Resulting from the sharing of three pairs of electrons (e.g., acetylene, C₂H₂).
The ability to form stable bonds with elements such as hydrogen, oxygen, and nitrogen allows carbon to participate in vast chemical networks essential for biological structures.
Relevance in Organic Chemistry
Carbon’s versatility in bonding is foundational in organic chemistry, forming complex molecules that are crucial for biological processes. Its four bonding electrons enable the formation of numerous functional groups such as alcohols, acids, and esters. Understanding its electronic configuration helps chemists predict how these compounds will react under various conditions, which is vital for applications in pharmaceuticals and biotechnology.
Influence in Material Science
The electronic configuration of carbon underlies its applications in material science, particularly in the development of allotropes such as graphite, diamond, and graphene. Each allotrope showcases different properties due to the variations in atomic arrangement and bonding. For instance, the strong covalent bonds in diamond lead to its exceptional hardness, while the layered structure of graphite allows for electrical conductivity and lubrication applications.
Conclusion and Key Takeaways
Summary of Carbon’s Electron Configuration
In summary, the electron configuration of carbon, represented as 1s² 2s² 2p², lays the groundwork for understanding its chemical behavior and the formation of various compounds. The placement of electrons across orbitals defines carbon’s bonding capacity, allowing it to participate in diverse structural frameworks.
Future Research Directions
Future research into the electron configurations of various elements, including carbon, provides opportunities to discover new materials and chemical processes. Areas such as quantum chemistry, nanotechnology, and materials science are particularly poised for advancements stemming from a deeper understanding of electronic structures.
Final Thoughts on Learning Chemistry
Understanding electron configurations is fundamental to chemistry, impacting everything from molecular design to industrial applications. As students and professionals navigate the vast field of chemistry, a solid grasp of essential concepts like electron configuration will prove invaluable, solidifying their knowledge and enhancing their capabilities in scientific inquiry and innovation.