Introduction to Electron Configuration
What is Electron Configuration?
Electron configuration is a fundamental concept in chemistry that describes the distribution of electrons in an atom. This configuration is crucial for understanding how atoms interact and bond with each other to form molecules. Each electron shell has a specific capacity for electrons, and the way these electrons are arranged determines the atom’s chemical properties and behavior.
The notation used for electron configuration involves the principal quantum number and the type of orbital (s, p, d, f). Electrons fill atomic orbitals in a specific order dictated by the principles of quantum mechanics, making this topic essential for anyone studying chemistry. For instance, the carbon electron config is a great illustration of these principles in action.
The Importance of Carbon in Chemistry
Carbon is often referred to as the backbone of life. With an atomic number of six, it is the fourth most abundant element in the universe by mass. Carbon’s unique ability to form stable bonds with many elements allows it to serve as the foundation for many molecules that constitute living organisms, including proteins, nucleic acids, carbohydrates, and lipids. Understanding carbon’s electron configuration is therefore crucial for grasping the complexities of organic chemistry and biochemistry.
Overview of Electron Shells
Electron shells are layers around the nucleus of an atom where electrons are likely to be found. Each shell corresponds to a principal energy level and is further divided into subshells that hold different types of orbitals. The first shell can hold up to two electrons, while the second can accommodate up to eight, and so forth. This arrangement is critical in determining the atom’s stability and reactivity, especially for elements like carbon that are central to many chemical processes.
Detailed View of Carbon Electron Configuration
Standard Notation of Carbon Electron Config
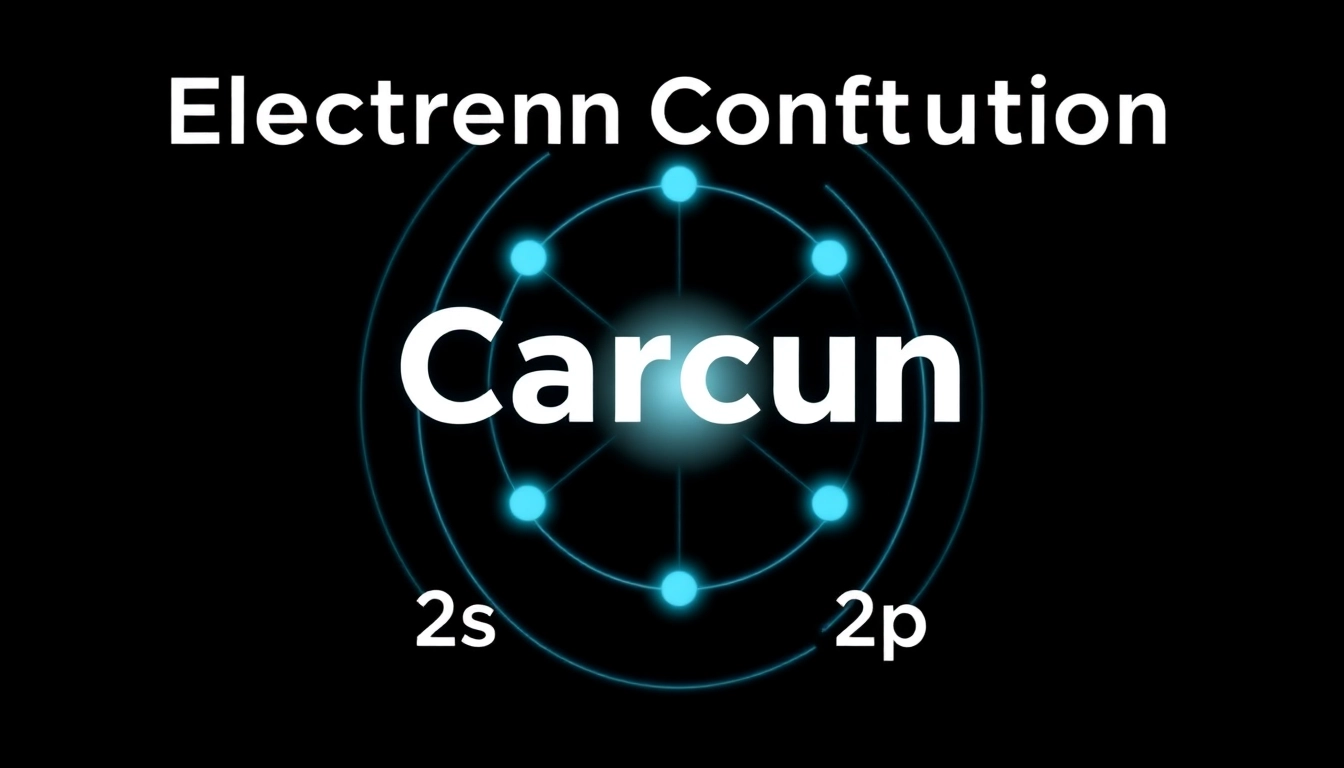
The standard electron configuration for carbon (C) is represented as 1s² 2s² 2p². This notation indicates that carbon has a total of six electrons: two in the first energy level (1s) and four in the second level (two in 2s and two in 2p). This arrangement leads to carbon’s tetravalency, allowing it to form four covalent bonds with other atoms, crucial for organic chemistry and the formation of complex molecules.
Visualizing Carbon’s Electron Shells
Visual aids such as orbital diagrams can be invaluable for understanding electron configurations. For carbon, the first shell is filled with two electrons (1s²), while the second shell has four electrons distributed across the 2s and 2p orbitals. The 2s orbital contains two electrons, while the two 2p orbitals can each hold one electron. This distribution contributes significantly to carbon’s ability to form diverse chemical structures, such as chains and rings.

Comparison with Other Elements
When compared to other elements, carbon’s electron configuration highlights its versatility. For example, oxygen, which has an electron configuration of 1s² 2s² 2p⁴, typically forms two covalent bonds due to its six total valence electrons. In contrast, nitrogen (1s² 2s² 2p³) can form three bonds. These distinctions emphasize the importance of understanding electron configurations in predicting the bonding behaviors of different elements.
The Role of Electron Configuration in Chemical Properties
Impact on Bonding and Reactivity
Electron configuration plays a vital role in defining how atoms bond. The way electrons are arranged around the nucleus affects the atom’s electronegativity, ionization energy, and electron affinity—all of which influence how readily an atom will participate in a chemical reaction. Carbon’s four valence electrons give it a unique advantage, allowing it to form stable covalent bonds with other elements, including itself, leading to a vast array of organic compounds.
Application in Organic Chemistry
In organic chemistry, the unique electron configuration of carbon allows it to engage in hybridization—a process where atomic orbitals mix to form new hybrid orbitals for bonding. For instance, in methane (CH₄), carbon undergoes sp³ hybridization, creating four equivalent sp³ hybrid orbitals, each forming a bond with hydrogen. This is a fundamental process for understanding the formation of various organic molecules, from simple hydrocarbons to more complex biological compounds.
Electron Configuration and Periodicity
The position of carbon in the periodic table directly correlates with its electron configuration. As we move across periods and down groups in the periodic table, trends in their properties can be attributed to changes in electron configuration. Periodicity helps chemists predict behavior in bonding, stability, and reactivity, with carbon’s unique properties explaining its central role in the chemistry of life.
Common Misconceptions about Carbon Electron Configuration
1s, 2s, 2p Differences Explained
One common misconception is that all orbitals are filled equally before moving to the next level. However, electron configuration follows specific rules such as the Aufbau principle, Pauli exclusion principle, and Hund’s rule. In carbon’s case, the 1s orbital is filled first, followed by the 2s, and then the 2p orbitals, reflecting the principles governing electron configuration.
Hybrids vs Non-Hybrids
Another misconception pertains to hybridization. While carbon often hybridizes its orbitals to maximize bonding efficiency, in many contexts it is useful to consider both hybridized and non-hybridized states. For example, when discussing carbon in its elemental form (e.g., graphite), the distinction between hybrid and standard configurations is critical for understanding its chemical and physical properties.
Why 1s² 2s² 2p²?
The electron configuration 1s² 2s² 2p² represents the most stable arrangement for carbon’s six electrons, minimizing energy within the atom. This configuration allows carbon to engage in four bonds, facilitating the formation of complex biological molecules while remaining stable in its elemental form. Understanding this configuration helps demystify the functional chemistry of carbon and its derivatives.
Conclusion and Further Reading
Resources for Learning More about Electron Configurations
To deepen your understanding of electron configurations, a number of resources are available. Websites like Khan Academy and educational platforms such as Coursera offer courses on chemistry fundamentals, including detailed discussions on electron configurations and their applications.
Contact Information for Expert Help
For those seeking personalized instruction or further insights into chemistry, consider reaching out to a qualified chemistry tutor or science educator. Many universities also offer resources, such as student-led tutoring centers, where you can engage with peers and experts alike.
Quiz Yourself: Test Your Knowledge
To reinforce what you’ve learned about carbon and electron configurations, consider taking a quiz! Practicing with problems that require you to determine electron configurations for various elements or predict their chemical behavior can greatly enhance your understanding of the subject.