Basics of Electron Configuration
What is Electron Configuration?
Electron configuration is a representation of the arrangement of electrons in an atom’s electron shells and sub-shells. It is denoted by a series of numbers and letters indicating the distribution of electrons among various energy levels, orbitals, and subshells. Understanding this arrangement is vital for predicting and explaining an element’s chemical behavior, bonding, and reactivity. For instance, the electron arrangement of carbon illustrates how carbon’s six electrons occupy specific orbitals influencing its interactions with other elements.
The Importance of Electron Arrangement
The arrangement of electrons in an atom significantly impacts its physical and chemical properties. It determines how an element will react with others, including the types of bonds it can form and the energy levels required for these processes. For example, elements with similar electron configurations often exhibit similar chemical properties, leading to the classification of elements into groups and periods in the periodic table. Moreover, the unique electron configuration of carbon, which allows it to form four bonds, is fundamental to the vast diversity of organic compounds.
Key Terms in Electron Configuration
- Orbitals: Regions in an atom where there is a high probability of finding an electron.
- Energy Levels: The fixed distances from the nucleus where electrons can be found.
- Shells: Layers around the nucleus of an atom where electrons exist; they are labeled as K, L, M, N, etc.
- Subshells: Subdivisions of shells consisting of orbitals; labeled as s, p, d, and f.
- Valence Electrons: Electrons in the outermost shell, crucial for determining an atom’s bonding capabilities.
The Electron Arrangement of Carbon
Step-by-Step Configuration Explanation
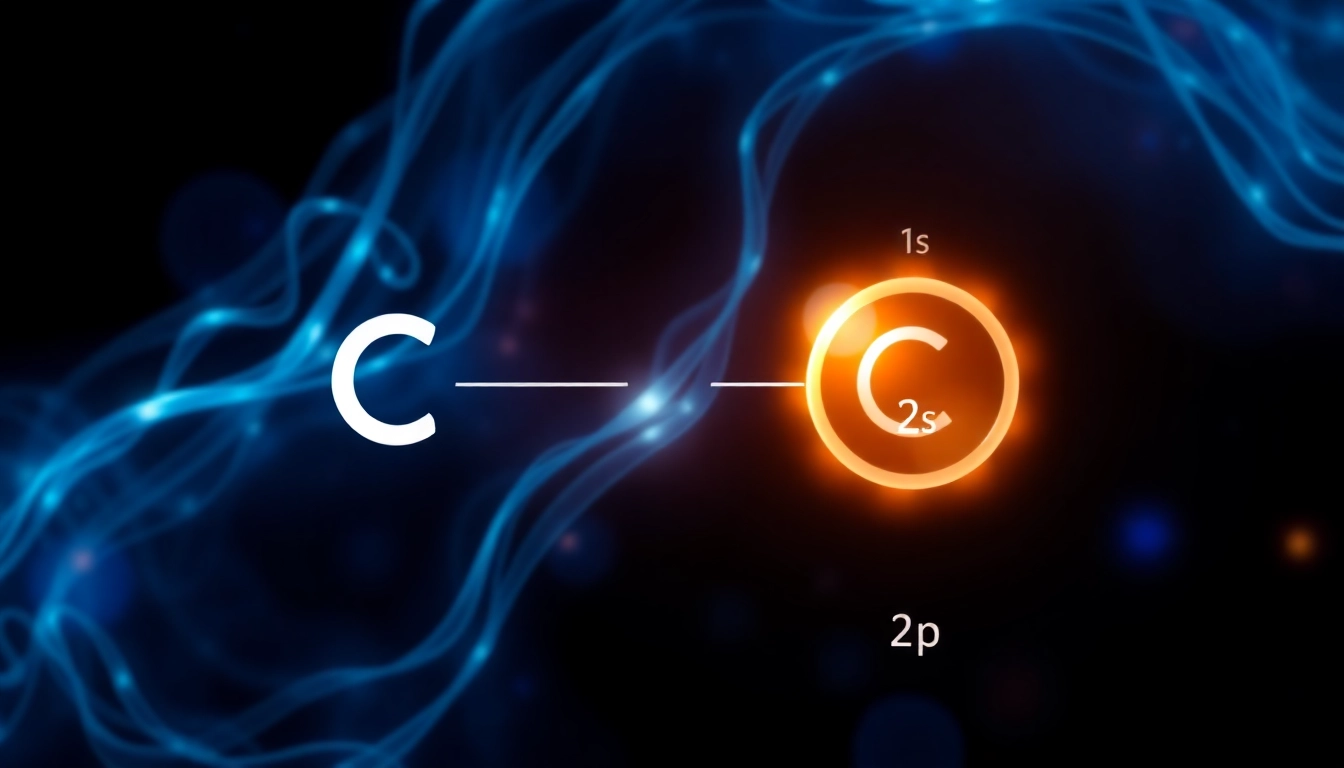
Carbon, with an atomic number of 6, has a total of 6 electrons. Its electron configuration is systematically determined by applying the principles of quantum mechanics. The first two electrons occupy the 1s orbital, followed by two electrons in the 2s orbital. This configuration can be written as:
1s2 2s2 2p2
In this format, ‘1s2‘ signifies that the first shell contains two electrons, while ‘2s2‘ indicates that the second shell’s s subshell also holds two electrons. The remaining two electrons are placed in the 2p subshell, resulting in an overall electron arrangement of 2,4. This distribution defines carbon’s characteristics, notably its tetravalency, or the ability to form four covalent bonds.
Orbital Diagrams for Carbon
Orbital diagrams visually represent electron configurations by depicting the various orbitals and the electrons occupying them. In the case of carbon:
The above diagram illustrates how electrons are arranged in the different orbitals according to the Hund’s rule and the Pauli exclusion principle. The 1s orbital is filled first, followed by the 2s, and finally the 2p orbitals, reflecting the energy levels and the capacity of each orbital.
Understanding Valence Electrons
Valence electrons are the electrons located in the outermost shell and play a crucial role in bonding and chemical reactions. For carbon, the four valence electrons located in the 2s and 2p orbitals allow it to form various types of bonds—single, double, and even triple bonds—with other elements. The unique ability of carbon to bond with itself and other elements facilitates the formation of complex molecules, making it a fundamental building block for organic compounds.
Comparative Analysis of Carbon’s Electron Arrangement
Electron Configurations in the Periodic Table
The periodic table is organized based on the electron configurations of elements. Carbon, positioned in period 2 and group 14, shares similarities in its electron configuration with other elements in the same group. Analyzing patterns within the periodic table, one can see that elements in the same group often have similar outer electron arrangements, which leads to comparable chemical properties.
Comparison with Neighboring Elements
When contrasted with neighboring elements like boron and nitrogen, carbon’s electron arrangement shows distinct characteristics. Boron, with an electron configuration of 1s2 2s2 2p1, has three valence electrons, resulting in fewer bonding possibilities than carbon. On the other hand, nitrogen, with a configuration of 1s2 2s2 2p3, has five valence electrons, allowing it to form more complex molecules but with different bonding patterns compared to carbon. This variation is critical for understanding the versatility of carbon in chemistry.
Role in Chemical Reactivity
The electron arrangement of carbon plays a pivotal role in its chemical reactivity. The four valence electrons are crucial for forming covalent bonds, allowing carbon to interact with a variety of elements, including hydrogen, oxygen, and nitrogen. The hybridization of these valence orbitals enables carbon to adopt different geometries and bond structures, crucial for the creation of diverse organic compounds. e.g., hydrocarbons, carbohydrates, and proteins, all relying on the intricate electron arrangements of carbon.
Applications of Carbon’s Electron Configuration
Implications in Organic Chemistry
The electron configuration of carbon is vital in organic chemistry, where the ability to form stable covalent bonds results in a vast array of organic compounds essential for life. Carbon’s tetravalency enables it to serve as a backbone for various functional groups in organic molecules, influencing their reactivity and properties. Understanding this configuration is fundamental for chemists engaged in synthesizing new compounds and exploring metabolic pathways.
Influence on Bonding and Structures
Carbon’s electron arrangement influences how it bonds with other elements and the resulting molecular structures. The ability of carbon atoms to hybridize their orbitals—forming sp, sp2, and sp3 hybridizations—leads to diverse molecular shapes like linear, trigonal planar, and tetrahedral structures. The bond angles and lengths further dictate the behavior of various organic molecules and their interactions with biological systems.
Real-World Examples and Case Studies
Case studies reflecting the implications of the electron arrangement of carbon encompass various real-world applications. For instance, the structure of glucose, a simple sugar composed of carbon, hydrogen, and oxygen, demonstrates how carbon’s valence electrons create ring structures central to metabolic processes. Another example is the study of carbon-based nanomaterials, where the unique bonding characteristics impart remarkable strength and conductivity, paving the way for advancements in material science.
Advanced Concepts in Electron Configuration
Understanding Hybridization
Hybridization is a concept central to understanding how carbon and other elements form covalent bonds. By merging different atomic orbitals, hybridization allows for new orbital formations, which in carbon’s case can lead to versatile bonding geometries. For example, in methane (CH4), the sp3 hybridization results in a tetrahedral structure, while in ethylene (C2H4), the sp2 hybridization produces a planar configuration.
The Impact of Electron Arrangement on Molecular Shape
The arrangement of electrons influences the molecular shape, which in turn affects properties such as polarity, reactivity, and phase. Carbon’s various hybridization states exemplify this; the three-dimensional arrangements of atoms in space are intrinsically tied to the electron configuration. Techniques such as X-ray crystallography reveal these three-dimensional structures, enhancing our understanding of molecular interactions in chemistry.
Future Trends in Electron Configuration Research
As our understanding of electron configuration deepens, future research is likely to focus on materials engineering, quantum computing, and nanotechnology. Advancements in computational methods are also expected to refine our grasp of electron dynamics, which will play a role in materials development and innovative applications in pharmaceuticals, energy storage, and environmental solutions. These emerging fields will hinge on an enhanced understanding of electron arrangements, particularly for versatile elements like carbon.