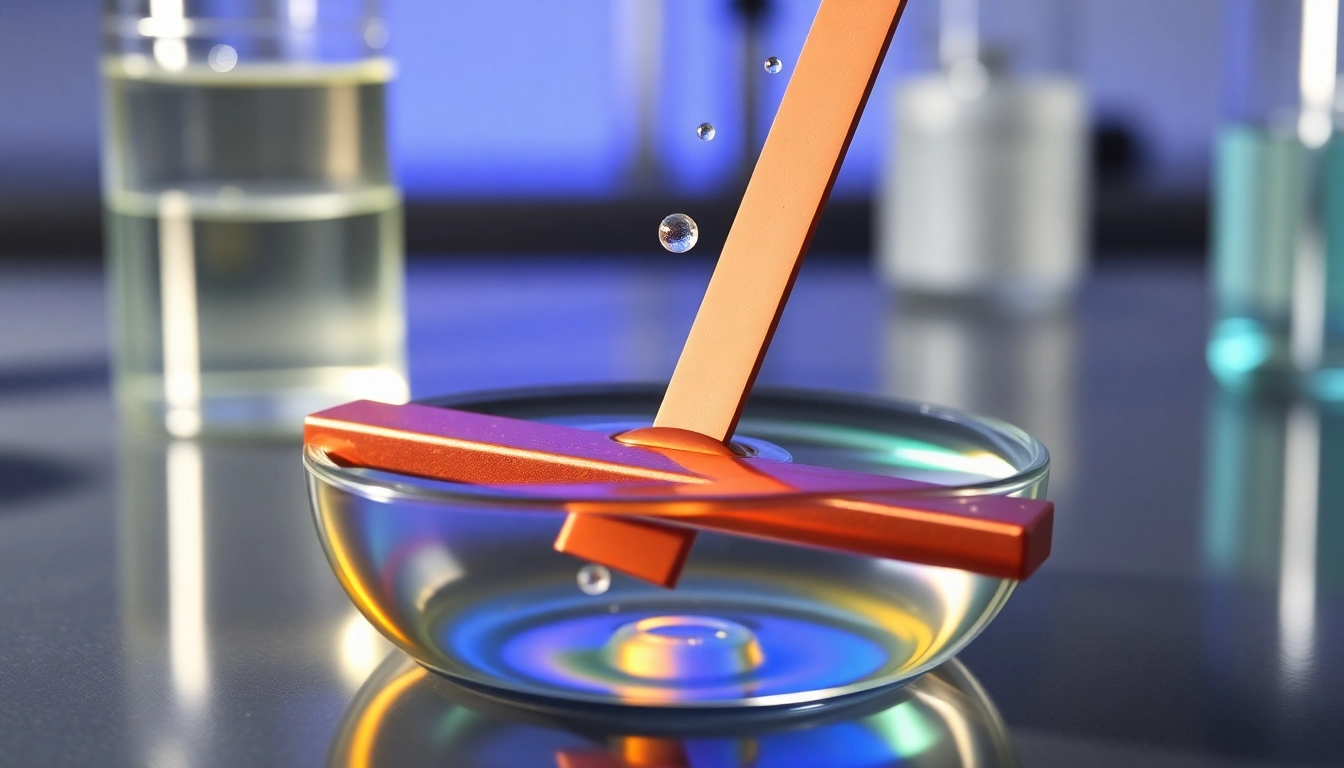
What is a Metal Displacement Reaction?
Definition of Metal Displacement Reaction
A metal displacement reaction, also known as a single displacement reaction, occurs when a more reactive metal replaces a less reactive metal in a compound. This type of reaction can be represented in the general formula:
A + BC → AC + B
In this equation, A is a more reactive metal that displaces B, a less reactive metal, from the compound BC, resulting in a new compound AC and the liberated element B. Displacement reactions are fundamental to understanding the behavior of metals and their interactions in various chemical processes.
Key Characteristics of Displacement Reactions
- Reactivity of Metals: The driving force behind metal displacement reactions is the reactivity series, which ranks metals based on their reactivity. A more reactive metal will always displace a less reactive metal from its compound.
- Element Conservation: In displacement reactions, the number of atoms remains constant before and after the reaction, conserving mass.
- Single Product Formation: Typically, a single product is formed in a metal displacement reaction, represented as a solid metal or a new ionic compound in solution.
- Energy Changes: Most metal displacement reactions are exothermic, releasing energy, primarily when they occur in aqueous solutions.
Importance in Chemical Reactions
Metal displacement reactions are crucial in various fields, including metallurgy, the production of metals from ores, and in laboratory applications. Understanding these reactions helps chemists predict the outcomes of experiments and leads to the discovery of new materials. Furthermore, such reactions are employed in numerous industrial processes, including electroplating, purification of metals, and recycling. A clear understanding of the metal displacement reaction example is essential for students and professionals alike to comprehend these industrial applications.
Exploring Common Metal Displacement Reaction Examples
Classic Examples in Chemistry
To illustrate metal displacement reactions, consider these classic examples:
- Zinc and Copper Sulfate:
When a strip of zinc metal is placed into a copper(II) sulfate solution, zinc, being more reactive than copper, displaces copper and forms zinc sulfate. The chemical equation is:
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s) - Magnesium and Hydrochloric Acid:
Magnesium can displace hydrogen from hydrochloric acid, producing magnesium chloride and hydrogen gas:
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g) - Aluminum and Iron(III) Oxide:
In a thermite reaction, aluminum displaces iron from iron(III) oxide, resulting in molten iron and aluminum oxide:
2Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2Fe(l)
Real-life Applications of Metal Displacement
The practical applications of metal displacement reactions are extensive. Here are a few notable examples:
- Extraction of Metals: Displacement reactions play a vital role in extracting metals from their ores. For instance, aluminum is used to reduce aluminum oxide (Al₂O₃) in the Hall-Héroult process.
- Battery Technology: Many batteries rely on displacement reactions. For example, zinc-carbon batteries utilize zinc to displace ions in a cathode, generating electrical energy.
- Galvanization: In this steel protection method, zinc is electroplated onto iron, serving as a sacrifice that protects the underlying metal from rusting.
Comparative Analysis of Reactive Metals
The reactivity of metals varies significantly, which is essential in predicting displacement reaction outcomes. The reactivity series ranks metals from highest to lowest reactivity based on empirical data and experimental evidence:
- Very Reactive: Potassium, Sodium, Calcium
- Reactive: Magnesium, Aluminum, Zinc
- Moderately Reactive: Iron, Nickel, Tin
- Less Reactive: Lead, Copper, Silver, Gold
This hierarchy indicates that potassium can displace all listed metals, while gold, being one of the least reactive, cannot displace any. Understanding this hierarchy allows chemists to predict displacement reactions and their outcomes effectively.
The Science Behind Metal Displacement
Reactivity Series of Metals
The reactivity series is pivotal in classifying metal displacement reactions. It is traditionally arranged by how readily various metals lose electrons — the more easily a metal loses electrons, the more reactive it is. This series plays a crucial role in determining whether a metal will displace another in a reaction. The position of a metal in this series can provide insights into its likelihood of participating in a displacement reaction.
Role of Electropositivity in Reactions
Electropositivity refers to the tendency of an atom to lose electrons and form positive ions. Highly electropositive metals are more likely to undergo oxidation, making them prime candidates for displacement reactions. For instance, metals like sodium and potassium will readily replace metals like copper or silver due to their high electropositivity. Understanding this tendency provides a clearer overview of how displacement reactions progress.
Oxidation and Reduction in Displacement Reactions
In metal displacement reactions, oxidation and reduction occur simultaneously, a process known as redox reactions:
- Oxidation: This involves the loss of electrons by the metal that is displaced. For example, in the reaction between zinc and copper(II) sulfate, zinc is oxidized:
Zn(s) → Zn²⁺(aq) + 2e⁻Cu²⁺(aq) + 2e⁻ → Cu(s)The balance of these two processes is fundamental in understanding how and why displacement reactions occur, underpinning the basic principles of electrochemistry.
Conducting Metal Displacement Experiments
Step-by-Step Guide for Safe Experiments
Conducting metal displacement experiments can be both educational and fun. Follow these guidelines for a safe and effective setup:
- Prepare Materials: You will need two different metal pieces (e.g., zinc and copper), a suitable ionic solution (like copper(II) sulfate), and safety equipment including goggles and gloves.
- Set Up the Experiment: Pour the ionic solution into a beaker and ensure it’s stable on a flat surface.
- Insert the More Reactive Metal: Submerge the zinc strip into the solution and leave it immersed for a predetermined amount of time.
- Analyze Observations: Carefully observe any changes, such as color changes or the formation of a precipitate.
- Dispose Responsibly: Follow appropriate waste disposal protocols to ensure no environmental harm occurs.
Common Mistakes to Avoid During Experiments
When performing metal displacement reactions, several errors can lead to inaccurate results:
- Incorrect Metal Selection: Ensure the metals chosen have a significant difference in reactivity based on the established series to observe clear results.
- Contaminated Solutions: Mixed solutions can interfere with reactions; always use fresh, uncontaminated solutions.
- Improper Timing: Allow enough time for the reaction to occur fully; premature measurement may lead to inaccurate conclusions.
Interpreting Results Accurately
Once your experiment is complete, analyze the results carefully. Look for key indicators such as:
- The formation of solid metal (precipitate).
- Color changes in the solution indicating a chemical reaction.
- Gas evolution, which suggests the displacement and production of gases like hydrogen.
Recording these observations meticulously is essential for developing accurate conclusions and further understanding the behavior of metals and their interactions.
Frequently Asked Questions about Metal Displacement Reactions
Common Queries and Clarifications
To aid learners and hobbyists, here are some frequently asked questions regarding metal displacement reactions:
- What is the difference between single and double displacement reactions? A single displacement reaction involves one element displacing another, while in a double displacement reaction, ions or atoms exchange places between two compounds.
- Can all metals participate in displacement reactions? No, only metals that are more reactive can displace others from their compounds. For example, hydrogen cannot be displaced from its compounds by metals like gold.
- How can I predict the outcome of a displacement reaction? By consulting the reactivity series, and identifying the more and less reactive metals involved in the reaction.
Additional Resources for Further Learning
For those interested in expanding their knowledge beyond this article, there are several resources available:
- GeeksforGeeks has numerous examples and applications of displacement reactions.
- The BBC Bitesize site offers educational resources and revision materials for chemistry learners.
- Visit MEL Chemistry for engaging experiments and insights into metal displacement reactions.
Real-world Implications of Metal Displacement
Understanding metal displacement reactions not only enhances academic knowledge but also has real-world implications. Industries rely heavily on these reactions for refining metal, producing alloys, and recycling materials. Knowledge of displacement reactions is essential for developing sustainable practices in metallurgy and chemical engineering, paving the way for innovation in materials science.