Introduction to the Electronic Structure of Carbon
Carbon stands as one of the most essential elements in the universe, second only to hydrogen and helium in terms of abundance in the cosmos. Its unique electronic structure allows for a diversity of bonding arrangements and interactions pivotal in both biological systems and industrial applications. Understanding the electronic structure of carbon is indicative of its capacity to form the backbone of organic chemistry, enabling everything from life to synthetic materials. In this article, we will explore the electron configuration of carbon, its role in chemistry, and how this fundamental knowledge can be applied in various scientific fields.
What is Electron Configuration?
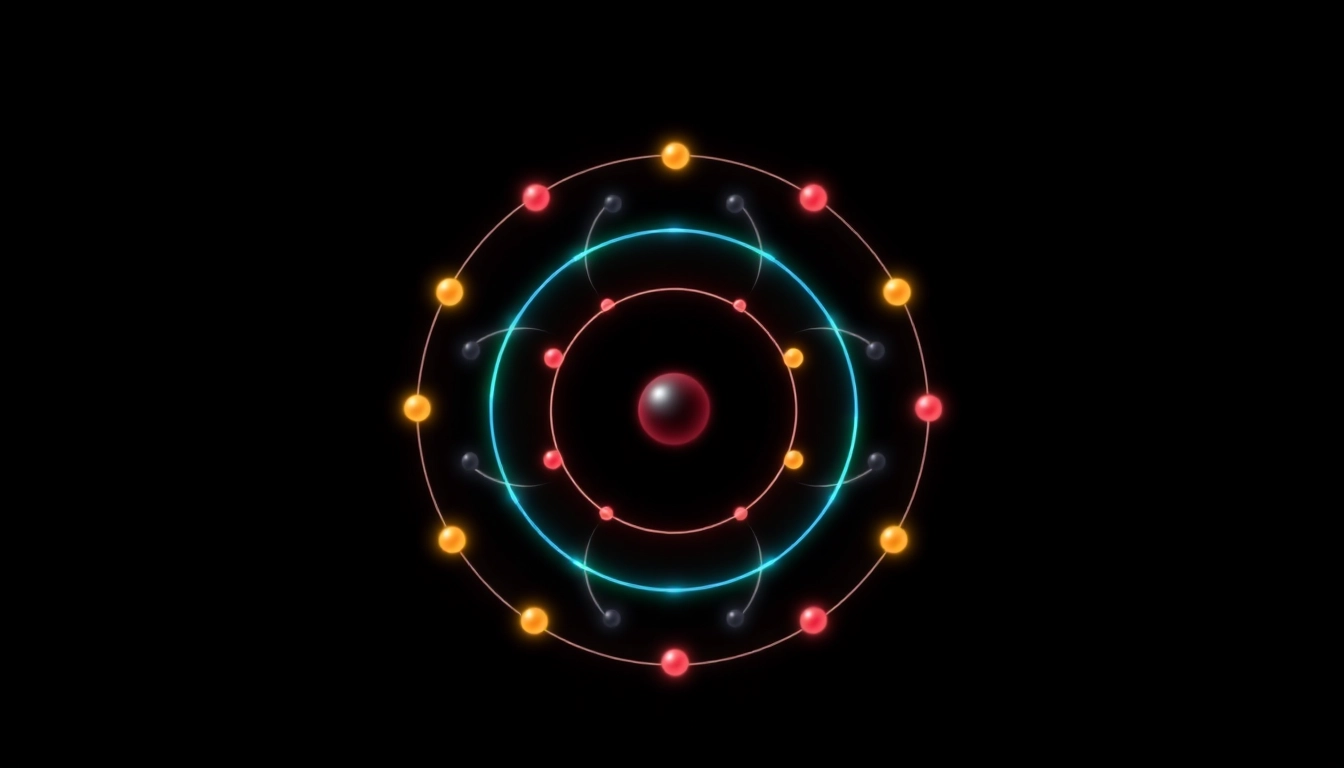
Electron configuration describes the distribution of electrons in an atom’s atomic orbitals. For carbon, which has an atomic number of 6, the electron configuration notation provides a shorthand method to indicate how these electrons are arranged. Each orbital can hold a specific number of electrons: the first shell (1s) can hold up to 2 electrons, while the second shell can hold 8 electrons, subdivided into the 2s and 2p subshells. The electron configuration of ground state carbon is typically denoted as 1s² 2s² 2p².
Importance of Carbon in Chemistry
Carbon’s electronic structure plays a critical role in its ability to form stable covalent bonds with various elements, including itself. This capacity leads to an extensive range of organic compounds, making carbon fundamental to life on Earth. Its tetravalent nature—with four valence electrons—allows carbon to participate in multiple bonding scenarios, including single, double, and triple bonds. The versatility in carbon bonding contributes to the complexity of organic molecules and biochemistry, influencing everything from DNA structure to synthetic polymers.
Basic Principles of Electronic Structure
The principles of electronic structure encompass several key concepts, including the Aufbau principle, Hund’s rule, and the Pauli exclusion principle. The Aufbau principle states that electrons occupy the lowest energy orbitals first. Hund’s rule posits that electrons will fill degenerate orbitals singly before pairing up, while the Pauli exclusion principle ensures that no two electrons can have the same set of quantum numbers. These principles govern how carbon and other elements stack their electrons in atomic orbitals, directly impacting their chemical properties.
Detailed Breakdown of Carbon’s Electron Configuration
Understanding Orbital Notation
In the case of carbon, the ground state electron configuration can be represented as 1s² 2s² 2p². This notation reveals that carbon has two electrons in the first energy level’s s orbital, and four electrons in the second energy level—two in the s orbital and two in the p orbitals. To further explain this, let’s consider each component:
1s²: The first shell (or principal energy level), where two electrons occupy the 1s orbital.2s²: In the second shell, two electrons fill the 2s orbital.2p²: Finally, within the same second shell, the two remaining electrons occupy two of the three available orientations in the 2p subshell.
The Significance of Valence Electrons
The four valence electrons in the outer shell of a carbon atom are crucial because they dictate how carbon interacts with other elements. These electrons can be shared to form covalent bonds, creating various stable molecules and influencing chemical reactivity. The arrangement allows carbon to form four covalent bonds, whether with hydrogen in hydrocarbons, with oxygen in carbon dioxide, or with itself in long chains and rings forming larger organic compounds.
Comparison with Other Elements
To understand carbon’s behavior more fully, it is beneficial to compare its electron configuration and valence electron arrangement with those of other elements. For instance:
- Hydrogen (H): With one electron, hydrogen can form a single bond. Its configuration is simply
1s¹. - Oxygen (O): With six electrons arranged as
1s² 2s² 2p⁴, oxygen requires two additional electrons to complete its octet, allowing it to form two bonds. - Nitrogen (N): Nitrogen, with five electrons (
1s² 2s² 2p³), forms three bonds by sharing its three unpaired electrons.
The versatility of carbon in forming tetravalent bonds distinguishes it from many other elements and is key to the structure of organic compounds.
How the Electronic Structure Influences Carbon’s Chemical Properties
Bonding and Reactivity
Carbon’s ability to form strong covalent bonds is derived from its electronic structure. The four valence electrons enable carbon to act as a versatile building block in organic chemistry. Because it can form stable bonds with itself and a variety of other elements, carbon can create chains, rings, and networks that lead to complex structures like carbohydrates, proteins, and nucleic acids. This flexibility also plays a role in the stability of organic compounds, influencing their reactivity under different conditions.
Role in Organic Chemistry
Carbon’s electronic structure is foundational in organic chemistry, defining the chemistry of compounds containing carbon. The numerous possible arrangements of carbon atoms and the functional groups that can attach to them lead to a vast array of organic materials. This includes everything from fuels and pharmaceuticals to polymers and natural products. Carbon’s capability to form multiple bonds enhances its presence in biomolecules and synthetic compounds, which is essential in the development of new materials and drug discovery.
Implications for Material Science
The unique properties stemming from carbon’s electron configuration also have significant implications in material science. For instance, diamond and graphite both consist of carbon atoms but display starkly different physical properties due to the arrangement of these atoms and the type of bonding involved. Diamond, with a tetrahedral arrangement of carbon atoms, is incredibly hard, while graphite, composed of layered planes of carbon atoms, is soft and useful as a lubricant and in batteries. Understanding these differences allows scientists to engineer materials with specific properties beneficial in applications ranging from electronics to construction.
Visual Representations and Diagrams
Orbital Diagrams Explained
To visually represent the electron configuration of carbon, orbital diagrams are invaluable. In these diagrams, the number of electrons is depicted with arrows, highlighting their spins and arrangement in orbitals. For carbon, you would have two arrows in the 1s orbital paired, two in the 2s orbital paired, and two single arrows in the 2p orbitals indicating their unpaired status. This visual can aid in understanding electron sharing during bond formation.
Graphical Depictions of Electron Configurations
Graphical representations, such as energy level diagrams, assist in conveying the relative energies of the orbitals. Carbon’s configuration can be illustrated in various charts showing the filling order and energy levels that define its reactivity. Such depictions are crucial in teaching and comprehending complex chemistry topics as they lay a more intuitive groundwork for further study.
Animations of Electron Behavior
In recent years, animations have become popular for demonstrating the dynamics of electron behavior. These visual tools can depict how electrons are involved in bonding and reactivity, portraying the fluid nature of electron sharing and movement within an atom or compound. They enhance the learning experience, particularly for students, by presenting challenging concepts in an engaging and easily digestible way.
Conclusion and Further Reading on Electron Configuration
Summary of Key Points
In conclusion, understanding the electronic structure of carbon is paramount to grasping the principles of chemistry that govern organic compounds and materials. With its unique electron configuration of 1s² 2s² 2p², carbon exhibits substantial versatility, forming a plethora of compounds essential for life and technology. Its fascinating ability to bond with itself and other elements propels it to the forefront of scientific exploration.
Recommended Resources for Additional Learning
For those interested in expanding their knowledge about the electronic structures and configurations, the following resources offer valuable insights:
- Electron Configuration for Carbon (C) – TerpConnect
- Electronic Structure and Atomic Orbitals – Chemguide
- 2.2: Electron Configurations – Chemistry LibreTexts
Future Trends in Carbon Research
As the scientific community continues to explore the electron configuration of carbon, future research may unlock new applications in nanotechnology, materials science, and renewable energy. Understanding carbon’s unique properties at the molecular level could pave the way for innovations in carbon capture technology and sustainability, further emphasizing the need to deepen our comprehension of this fundamental element.