Introduction to Carbon Electronic Structure
Understanding the carbon electronic structure is fundamental to the fields of chemistry, physics, and materials science. Carbon, the sixth element in the periodic table, has unique properties that are largely attributable to its electron configuration. This article will explore carbon’s electronic structure, examine its various configurations, analyze its significance in organic chemistry, and delve into advanced topics such as orbital hybridization and quantum mechanics.
What is Electron Configuration?
Electron configuration describes the distribution of electrons in an atom’s atomic orbitals. As we delve deeper into the atomic structure, we can understand that electrons reside in specific energy levels or shells, each capable of holding a limited number of electrons. This configuration is crucial in determining how an atom interacts and bonds with others, thus influencing the chemical and physical properties of an element.
Importance of Carbon in Chemistry
Carbon stands as a cornerstone in organic chemistry and biochemistry due to its ability to form stable covalent bonds with a variety of elements, including hydrogen, oxygen, nitrogen, and itself. Its versatile bonding characteristics enable it to create a vast array of complex molecules essential for life, such as proteins, DNA, and carbohydrates. Understanding carbon’s electronic structure is fundamental in elucidating its reactivity and the mechanisms of organic reactions.
Overview of the Carbon Atom
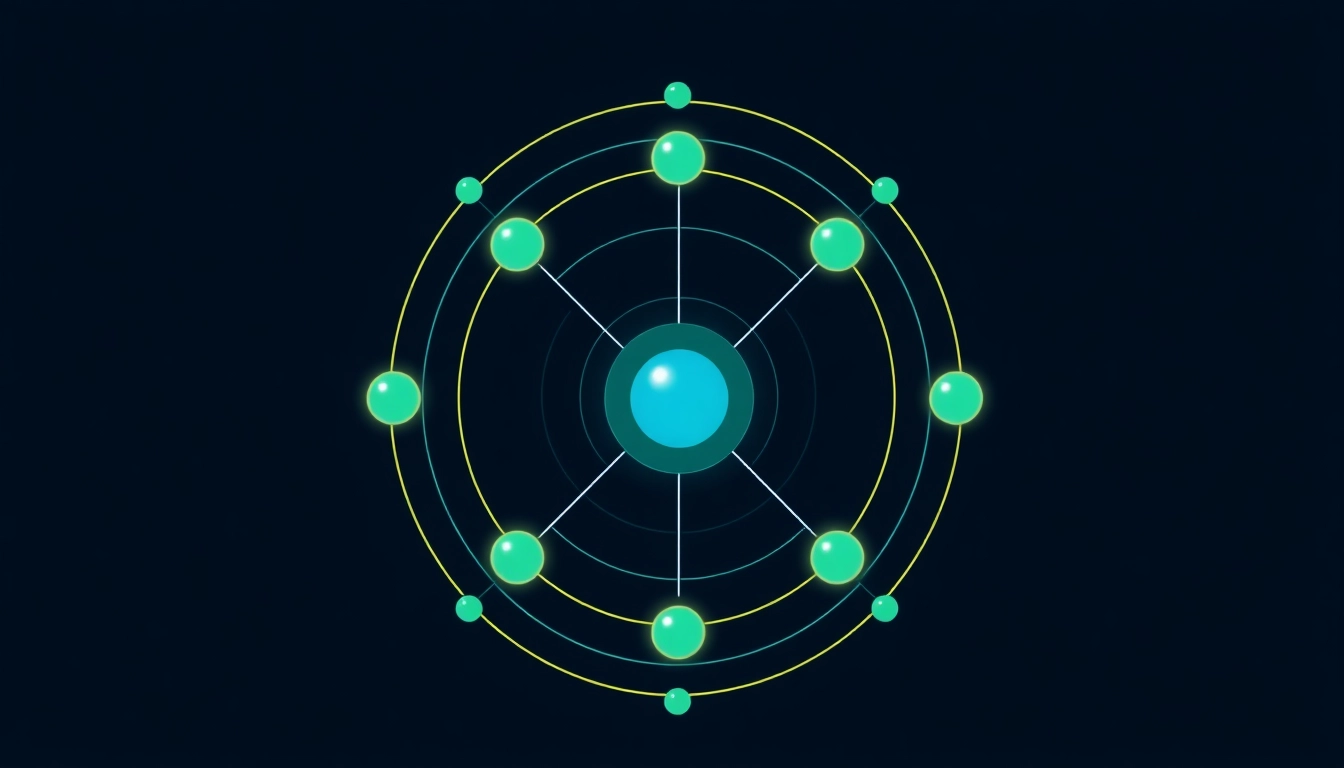
A carbon atom has six protons and six electrons, and its ground state electron configuration is expressed as 1s² 2s² 2p². This establishes two occupied energy levels: the first shell has two electrons in the 1s orbital, while the second shell contains four electrons, divided between the 2s and 2p orbitals. The arrangement of these electrons not only determines how carbon interacts chemically but also explains its unique properties in various compounds.
Basics of Carbon’s Electron Configuration
Ground State Electron Configuration of Carbon
The ground state electron configuration for a neutral carbon atom can be expressed as follows:
- 1s²: Two electrons in the first shell.
- 2s²: Two electrons in the second shell’s s orbital.
- 2p²: Two of the four remaining electrons fill the 2p orbital, with the capability of forming bonds.
In total, the configuration is often written as [He] 2s² 2p², where [He] indicates the electron configuration of helium, the preceding noble gas.
Shells and Subshells Explained
Carbon’s electrons occupy different shells and subshells based on their energy levels. The two primary shells—K (1st) and L (2nd)—each have subshells that follow a specific filling order:
- Electrons fill the 1s subshell first.
- Next, both the 2s subshell and then the 2p subshell get filled according to the Aufbau principle.
This arrangement is crucial as it highlights the availability of energy levels for bonding and chemical reactions.
Valence Electrons and Their Roles
Carbon has four valence electrons (two in the 2s and two in the 2p subshells), which play a significant role in chemical bonding. The number of valence electrons determines an atom’s bonding capacity and reactivity. For carbon, these four valence electrons allow it to form four covalent bonds with other atoms, making it exceptionally versatile and capable of forming single, double, or triple bonds with various elements.
Understanding Carbon Electronic Structure Variants
Excited State Configurations of Carbon
When a carbon atom absorbs energy, one of its electrons may be promoted to a higher energy orbital, resulting in an excited state configuration. For example, one of the 2s electrons can be promoted to a higher 2p orbital, leading to the configuration of 1s² 2s¹ 2p³. Excited state configurations are vital in understanding phenomena such as spectral emission and chemical reactivity under certain conditions.
Carbon Ions and Their Electron Configurations
Carbon can also exist in ionic forms, such as the carbon cation (C⁺) and anion (C⁻). The electron configurations for these ions vary slightly. For a carbon cation, one electron is lost from the 2p orbital, resulting in a configuration of 1s² 2s² 2p¹. Conversely, for a carbon anion, an extra electron is gained, leading to the configuration of 1s² 2s² 2p³. Understanding these configurations is critical in various chemical reactions and processes.
Common Isotopes and Their Electron Structures
Carbon has three primary isotopes: Carbon-12, Carbon-13, and Carbon-14. Though they differ in neutron count, all three isotopes preserve the same electron arrangement due to their identical electron configurations. These isotopes have unique applications; for example, Carbon-14 is used in radiocarbon dating, while Carbon-12 and Carbon-13 are often employed in studies of metabolic pathways in biological systems.
Applications of Carbon’s Electronic Structure
Carbon’s Role in Organic Chemistry
In organic chemistry, carbon’s ability to form stable bonds with other carbon atoms and various elements leads to the formation of complex macromolecules. This chemical versatility is pivotal in forming a vast range of organic compounds, including hydrocarbons, alcohols, acids, and more. Understanding the electronic configuration of carbon allows chemists to predict how different compounds will react and interact in various conditions.
Understanding Hybridization in Carbon Compounds
Hybridization is a key concept in understanding the bonding behavior of carbon atoms. In carbon compounds, hybridization occurs when atomic orbitals combine to form new hybrid orbitals, which can help describe the geometry of molecular structures. For instance, in methane (CH₄), the 2s and three 2p orbitals hybridize to create four equivalent sp³ hybrid orbitals that orient themselves in a tetrahedral geometry, leading to the characteristic shape of the molecule.
Examining Reactions Involving Carbon
Carbon’s electronic structure heavily influences its reactivity in various chemical reactions. For example, the formation of double bonds involves the sharing of two pairs of electrons between carbon atoms, leading to more stable compounds such as alkenes. Additionally, understanding the electronic structure helps predict the mechanisms of reactions—ranging from electrophilic additions in unsaturated molecules to nucleophilic substitutions and eliminations in organic chemistry.
Advanced Concepts in Carbon Electronic Structure
The Significance of Orbital Hybridization
Orbital hybridization is a sophisticated concept that combines atomic orbitals to create new orbitals with distinct shapes and energy levels. In carbon, hybridization can lead to various geometrical arrangements in molecular structures: sp, sp², and sp³ hybridizations correlate with linear, trigonal planar, and tetrahedral geometries, respectively. This concept is integral in understanding reaction mechanisms, molecular stability, and spectral properties.
Quantum Mechanics and Carbon Structures
The quantum mechanical model provides a robust framework for understanding atomic and molecular structures. It emphasizes wave-particle duality and the probabilistic nature of electron positions, which leads to various interpretations of carbon’s behavior in different contexts. For example, quantum mechanics elucidates how carbon forms pi bonds through the overlap of p orbitals, crucial for understanding the structure of aromatic compounds.
Recent Research on Carbon Atomic Properties
Ongoing research continues to explore the nuanced properties of carbon atoms and their electronic structures. Current studies focus on innovations in materials science, particularly in the development of carbon-based nanomaterials, such as graphene and carbon nanotubes, which exhibit remarkable electrical, mechanical, and thermal properties. Understanding the electronic structure of these materials is essential for optimizing their applications in electronics, energy storage, and nanotechnology.