Understanding Electron Configuration
The Basics of Electron Configuration
Electron configuration refers to the distribution of electrons in an atom’s orbitals. Understanding this distribution is crucial, as it governs both an atom’s reactivity and its position on the periodic table. The arrangement of electrons is based on principles such as the Aufbau principle (electrons fill from the lowest energy level up), Pauli exclusion principle (no two electrons in the same atom can have the same set of quantum numbers), and Hund’s rule (every orbital in a subshell is singly occupied before any orbital is doubly occupied).
Why Electron Configuration Matters
The study of electron configuration is fundamental in chemistry and physics, as it can help predict how atoms interact with one another. Elements with similar electron configurations exhibit similar chemical properties, allowing scientists to categorize and understand elements systematically. For instance, alkali metals have a single electron in their outer shell and thus are highly reactive.
How to Determine Electron Configuration for Elements
To determine the electron configuration for any element, one must consider its atomic number, which indicates the number of electrons present in a neutral atom. The electrons are filled into orbitals following the aforementioned rules. A great resource for understanding the basic principles of electron configuration is to practice using the periodic table.
The Electron Configuration for Carbon
The Significance of Carbon’s Configuration
Carbon is the sixth element on the periodic table, denoted by the symbol C. With an atomic number of 6, it has a total of 6 electrons. This configuration is pivotal as carbon serves as the backbone of organic chemistry, forming the base structure for a vast array of biological compounds, including proteins, carbohydrates, lipids, and nucleic acids. Moreover, carbon’s ability to form stable bonds with various elements is a fundamental characteristic that enables the complexity of life.
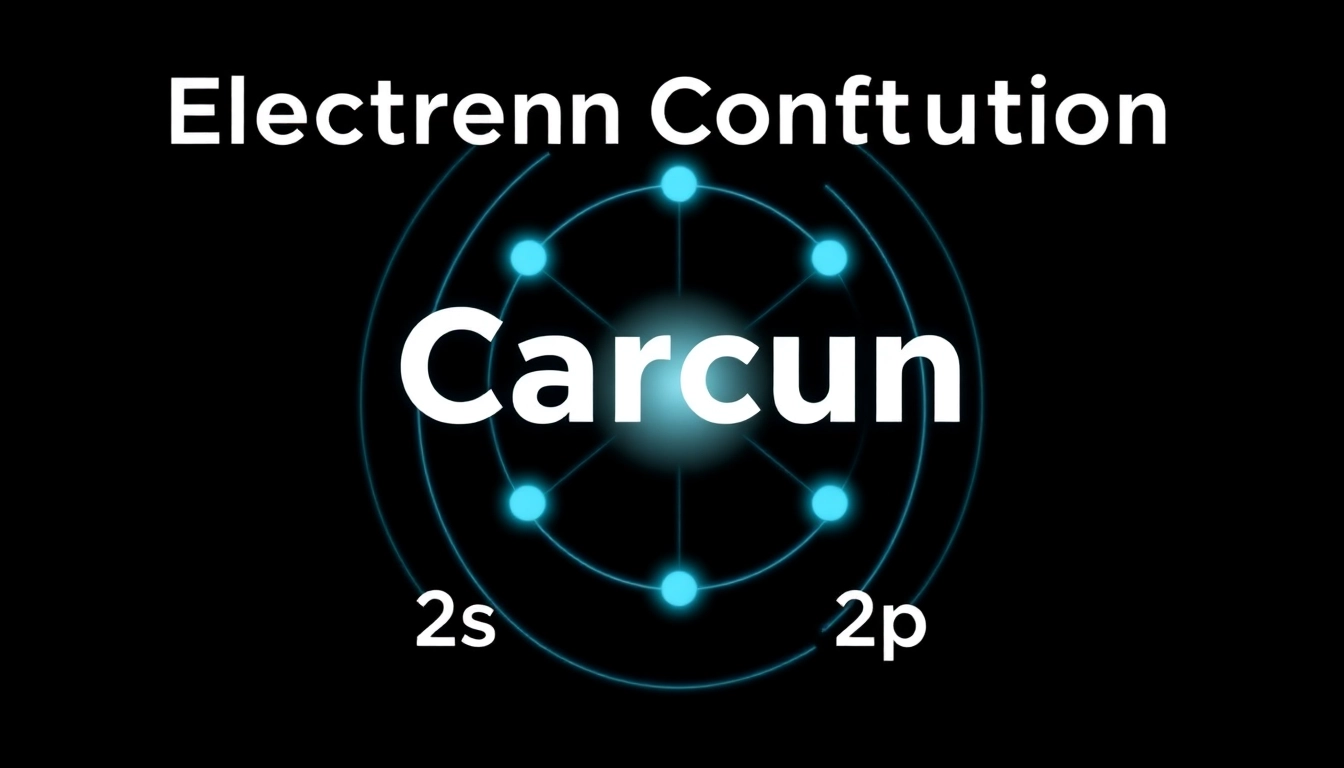
Notation Breakdown: 1s² 2s² 2p²
The electron configuration for carbon is typically written as 1s² 2s² 2p². This notation indicates that:
- The first two electrons occupy the 1s orbital (lowest energy level).
- The next two electrons fill the 2s orbital.
- The final two electrons are distributed in the 2p orbitals.
The electron configuration for carbon is significant in determining its bonding behavior and how it interacts with other elements.
Comparison with Other Elements
When comparing carbon’s electron configuration to other elements, we can see how it facilitates unique chemical properties. For example, oxygen has an electron configuration of 1s² 2s² 2p⁴. Oxygen’s additional unpaired electrons give it a high reactivity with carbon, leading to the formation of essential compounds like carbon dioxide. On the other hand, noble gases like neon, which has a complete outer shell (1s² 2s² 2p⁶), exhibit minimal reactivity due to their stable electron arrangements.
Visualizing Carbon’s Electron Configuration
Orbital Diagrams Explained
To visualize electron configuration, orbital diagrams can be enormously helpful. These diagrams allow for clear representation of how electrons are arranged within orbitals. Each box in the diagram represents an orbital, while arrows indicate the presence of electrons. Following Hund’s rule, each orbital in a subshell is first singly occupied before pairing occurs. An orbital diagram for carbon would display:
1s (↑↓), 2s (↑↓), and 2p (↑↑ __).
This visual aid helps facilitate understanding of both electronic arrangement and potential chemical bonding.
Using the Periodic Table for Visualization
The periodic table itself serves as an invaluable tool for understanding electron configuration. Elements are organized by increasing atomic number, which correlates with their electron configurations. Groups of elements that share similar configurations often exhibit analogous properties. By studying trends in the periodic table, we can predict an element’s reactivity, ionization energy, and other characteristics based on its position.
Interactive Tools and Resources
Various educational resources and interactive tools are available for better comprehension of electron configurations. Websites offering simulation tools allow users to visualize how electrons are populated in different elements. Online quizzes and flashcards can also reinforce understanding through active learning methods. Additionally, video explanations on platforms like YouTube often provide step-by-step tutorials for writing and interpreting electron configurations.
Applications of Carbon’s Electron Configuration
Carbon in Organic Chemistry
Carbon’s electron configuration is foundational in organic chemistry. The four valence electrons allow carbon to form up to four covalent bonds with other atoms, enabling the creation of complex molecules. This ability to bond in various configurations (single, double, and triple bonds) results in an extensive variety of organic compounds. For example, the structure of DNA, proteins, and carbohydrates is largely dependent on carbon bonding arrangements.
Impacts on Molecular Geometry
The geometry of molecules is determined by the electron configuration of the atoms involved. Carbon typically adopts tetrahedral geometry in compounds where it forms four bonds (as in methane, CH₄). This spatial arrangement is critical for understanding reaction mechanisms, molecular interactions, and the overall functionality of organic compounds. Similarly, the linear and planar configurations seen in compounds containing double and triple bonds respectively are a direct consequence of hybridization and orbital overlap.
Electron Configuration and Reactivity Patterns
Echoing its role in organic chemistry, carbon’s electron configuration significantly impacts its reactivity. The four outer electrons allow for diverse bonding and reactivity patterns, distinguishing carbon-based compounds from those composed of elements with fewer bonding capabilities, such as silicon or boron. Reactivity can also be predicted by examining the stability of awaiting physicochemical states based on the electron configuration, providing insights into reaction mechanisms, rates, and products.
Common Misconceptions About Electron Configuration
Differences in Notation
One common misconception surrounding electron configuration is the interpretation of different notations. For example, while the electron configuration for carbon is often expressed as 1s² 2s² 2p², variations may appear where different conventions might be applied. Understanding that both representations ultimately refer to the same distribution of electrons (albeit through different perspectives) is essential for clarity when studying chemistry.
Clarifying the Ground State vs. Excited State
Another area of confusion is the distinction between ground state and excited state electron configurations. In the ground state, electrons are arranged in the lowest energy positions possible, as seen in carbon’s stable configuration. However, when energy is added (e.g., through heat or light), electrons can transition to higher energy orbitals, resulting in excited state configurations. Understanding these transitions is crucial when discussing chemical reactions, spectroscopic properties, and behavior under various conditions.
Frequently Asked Questions
To enhance comprehension, here are some frequently asked questions regarding electron configuration:
- What is the electron configuration of elements other than carbon? Each element has a unique configuration based on its atomic number. For instance, oxygen (O) has the configuration 1s² 2s² 2p⁴, while nitrogen (N) is 1s² 2s² 2p³.
- Why does electron configuration matter in chemical reactions? The reactivity and bonding patterns of elements are influenced significantly by their electron configurations, impacting how they behave during chemical reactions.
- Can electron configuration change? Electron configurations can change when electrons are gained or lost (as in ionization), or when energy is absorbed allowing them to occupy higher energy states.