Introduction to Carbon and Its Importance
Carbon, the sixth element on the periodic table, is an essential building block of life. Its unique ability to form stable bonds with various elements enables it to play a critical role in the makeup of all known life forms. Understanding carbon’s properties, especially in terms of its c full electron configuration, is vital for grasping its behavior in various chemical processes.
What Is Carbon?
Carbon is a non-metal found abundantly on Earth, primarily in organic compounds, minerals, and gases. Its atomic number is 6, which means it contains six protons and, in its neutral state, six electrons. The structure of carbon allows for numerous forms, including diamonds and graphite, which exhibit vastly different properties despite having the same elemental composition.
The Role of Carbon in Life
This element is the cornerstone of organic chemistry, the study of carbon-containing compounds. Carbon’s versatility arises from its ability to form four covalent bonds with other atoms, enabling the construction of complex molecules essential for biological function, such as proteins, nucleic acids, carbohydrates, and lipids.
Overview of Electronic Configuration
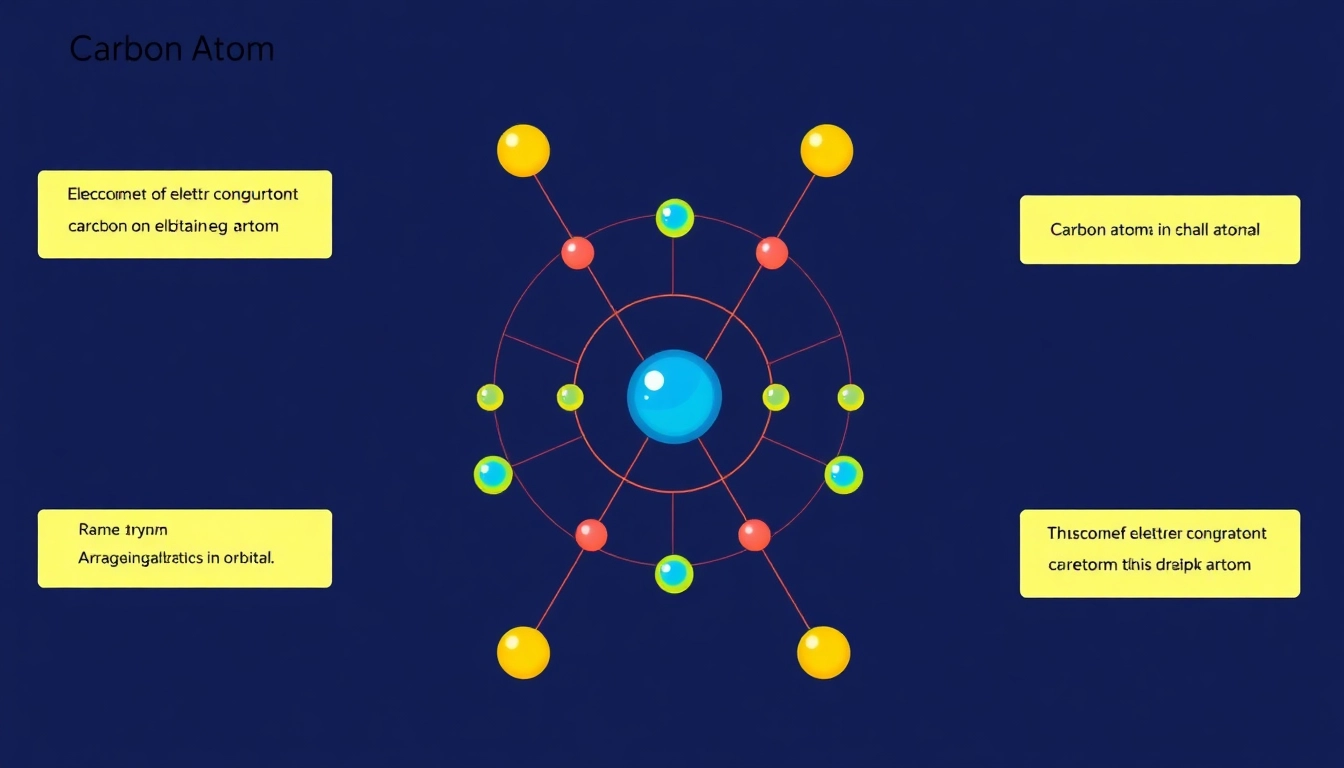
Electron configuration is the distribution of electrons in an atom’s orbitals. For carbon, the full electron configuration is crucial in understanding its reactivity and bonding behavior. The specific arrangement of electrons in carbon gives insight into its ability to form covalent bonds with a variety of elements, influencing everything from biological processes to material science.
Basics of Electron Configuration
What Is Electron Configuration?
Electron configuration describes the arrangement of electrons within different energy levels and orbitals around an atom’s nucleus. Each element has a distinct electron configuration that determines its properties, reactions, and stability. This configuration helps predict how an atom will interact with other atoms, forming molecules and compounds.
Principles of Electron Filling
Electrons obey specific rules when occupying orbitals, including:
- The Aufbau Principle: Electrons occupy the lowest energy orbitals first, filling up from the inner shell to outer shells.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins.
- Hund’s Rule: When electrons occupy degenerate orbitals (orbitals at the same energy level), one electron is placed in each orbital before pairing occurs.
Orbital Diagrams Explained
Orbital diagrams visually represent the arrangement of electrons in an atom. They illustrate how electrons fill subshells within an atom’s shells, using arrows to indicate individual electrons. For carbon, the orbital diagram displays the filling order for 1s, 2s, and 2p orbitals:
- 1s: ↑↓
- 2s: ↑↓
- 2p: ↑ ↑
This arrangement reflects the distribution of carbon’s electrons across its respective orbitals.
Full Electron Configuration of Carbon
Writing the C Full Electron Configuration
The full electron configuration for carbon can be noted as 1s² 2s² 2p². This notation indicates that carbon has:
- Two electrons in the 1s orbital
- Two electrons in the 2s orbital
- Two electrons in the 2p orbital
In a condensed format, this can be represented as [He] 2s² 2p², where [He] represents the electron configuration of helium, which has a complete shell structure.
The Significance of [He] 2s2 2p2
The significance of the electron configuration [He] 2s² 2p² lies in understanding carbon’s valence electrons, which are pivotal for its chemical properties. These four valence electrons are involved in carbon’s ability to form four covalent bonds, making it highly versatile in forming complex organic molecules.
This configuration allows carbon to engage in various bonding scenarios, from forming long chains in hydrocarbons to creating intricate structures in biomolecules, such as DNA and proteins.
Common Mistakes to Avoid
When studying electron configurations, common mistakes include:
- Inaccurately counting electrons or skipping orbitals
- Incorrectly applying Hund’s Rule
- Misunderstanding the significance of noble gas core notations
A solid comprehension of orbital filling principles will aid in preventing these mistakes and deepen the understanding of not only carbon but other elements as well.
Comparison with Other Elements
Differences Between Carbon and Oxygen Electron Configurations
Oxygen, with an atomic number of 8, has the electron configuration 1s² 2s² 2p⁴. Comparing this to carbon’s configuration, we see that oxygen has two additional electrons that fill two more of the 2p orbitals, increasing its electronegativity and reactivity compared to carbon. This reactivity is evident in the formation of water and other vital compounds where oxygen often acts as a strong oxidizing agent.
Why Carbon’s Configuration Is Unique
Carbon’s electron configuration is unique due to its ability to form various stable compounds and different allotropes. This element is capable of hybridization, which allows its orbitals to mix, forming new, equivalent orbitals that participate in bond formation. This hybridization is what enables the vast diversity of organic molecules. For example, carbon’s ability to hybridize its s and p orbitals allows for the formation of tetrahedral (sp³), trigonal planar (sp²), and linear (sp) geometries, leading to an extensive variety of molecular shapes and reactivities.
Isoelectric Elements and Their Configurations
Isoelectronic elements are those that have the same number of electrons or the same electron configuration. For instance, the negative ion of carbon (C⁻) has the electron configuration of 1s² 2s² 2p⁶, which is the same as neon (Ne). This property is significant as it demonstrates how electron configurations can indicate stability and bonding capabilities in various chemical contexts.
Applications and Implications of Carbon’s Electron Configuration
Understanding Carbon’s Reactivity
Carbon’s full electron configuration plays a crucial role in its reactivity. The arrangement of its valence electrons enables it to bond with a wide array of elements, including not just other carbon atoms, but also oxygen, nitrogen, hydrogen, and many others. This ability allows carbon to participate in countless chemical reactions and is foundational for the field of organic chemistry, where understanding reactivity is essential for synthesizing new compounds.
Electron Configuration in Organic Chemistry
Organic chemistry relies heavily on the understanding of carbon’s electron configuration to predict how compounds will interact. The presence of four valence electrons allows carbon to undergo various types of hybridization and bonding, leading to different functional groups like alcohols, ketones, and carboxylic acids. The stability and reactivity of these organic molecules can often be traced back directly to the underlying electron configuration of carbon and its derivatives.
Impact on Material Science and Nanotechnology
In material science and nanotechnology, carbon’s electron configuration is utilized to design materials with specific electrical, thermal, and mechanical properties. Carbon allotropes, such as graphene and carbon nanotubes, exhibit exceptional strength and conductivity, opening avenues for advancements in electronics, materials engineering, and nanotechnology. Understanding carbon’s electron configuration enables scientists and engineers to manipulate these materials at the molecular level, paving the way for innovative products and technologies.
Thus, studying the c full electron configuration of carbon is not only crucial from a theoretical standpoint but also has practical implications across various scientific disciplines.