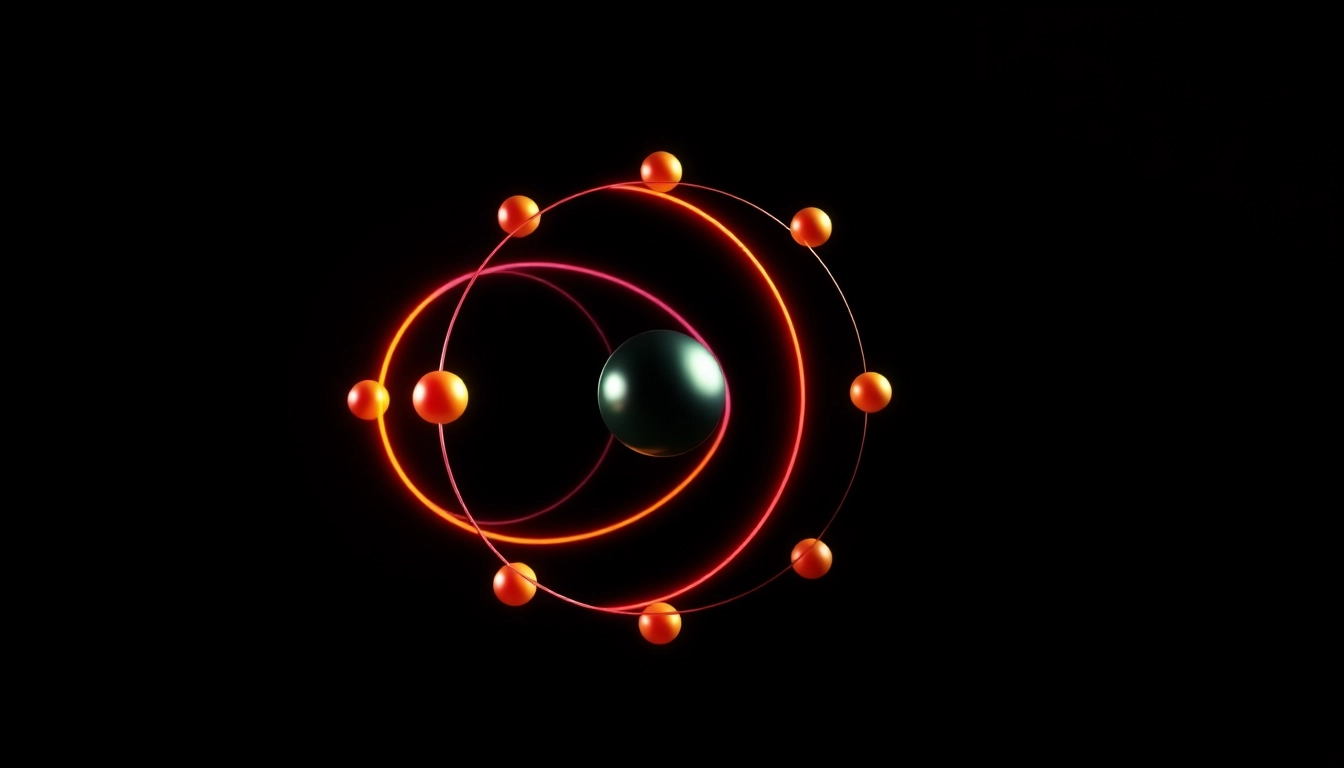
Introduction to Electron Configuration
Electron configuration is a fundamental concept in chemistry, describing how electrons are arranged in an atom’s atomic orbitals. Understanding the electron config carbon provides insights into the element’s behavior, reactivity, and overall properties, establishing a foundation for more advanced studies in both chemistry and materials science. Carbon, as one of the most essential elements in organic compounds, reveals much about structure and function across biological, physical, and chemical landscapes. This article delves deeply into the electron configuration of carbon, its implications, and its applications in various scientific fields.
What is Electron Configuration?
Electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. It is expressed using a series of notations that detail the energy levels, sublevels, and the number of electrons occupying each level. For example, in a neutral carbon atom, the electron configuration is written as:
1s2 2s2 2p2. This notation describes that carbon has two electrons in the first energy level’s s orbital (1s), two electrons in the second energy level’s s orbital (2s), and two electrons in the second energy level’s p orbital (2p).
Importance of Electron Configurations in Chemistry
Understanding electron configurations is crucial for predicting how atoms combine and interact. The arrangement of electrons is the primary determinant of an atom’s chemical properties, including its reactivity, bonding behavior, and the types of bonds it can form. For example, carbon’s unique ability to form four bonds (tetra-valence) due to its electron configuration allows for the vast complexity of organic molecules, which are fundamental to life.
The Basics of Electron Configurations and Atomic Structure
The structure of an atom consists of a nucleus containing protons and neutrons, surrounded by electrons that occupy various energy levels or shells. The distribution of electrons is governed by quantum mechanics and follows specific rules such as the Aufbau principle, Pauli exclusion principle, and Hund’s rule. A correct electron configuration reflects not only the number of electrons present but also their distribution according to these principles, resulting in a stable configuration.
The Electron Configuration of Carbon
Carbon (C), with an atomic number of 6, has six electrons distributed across the available atomic orbitals. Its electron configuration has significant chemical relevance, forming the backbone of many organic compounds.
Detailed Breakdown of Carbon’s Configuration
The electron configuration for carbon is written as 1s2 2s2 2p2. Each component represents:
- 1s2: The first two electrons occupy the 1s orbital. This orbital is the lowest energy level and can hold a maximum of two electrons.
- 2s2: The next two electrons are in the 2s orbital, which is also filled with a maximum of two electrons.
- 2p2: The final two electrons occupy the 2p orbital, which can hold up to six electrons. In carbon, these electrons are distributed in the 2p orbitals (2px and 2py) following Hund’s rule, each occupying separate orbitals before pairing.
Carbon’s Position in the Periodic Table
Carbon is located in Group 14 of the periodic table and holds the 4th period. Its position reflects its ability to form various chemical bonds due to the presence of four valence electrons (from its 2s and 2p orbitals). Carbon’s electronegativity (about 2.5 on the Pauling scale) and its unique bonding characteristics make it a keystone element in organic chemistry.
Comparison of Carbon Configuration to Other Elements
When compared to its neighbors in the periodic table, carbon’s electron configuration reveals significant differences that influence its chemistry. For example:
- Boron (B): Boron has an electron configuration of 1s2 2s2 2p1, which means it has only three valence electrons and forms three bonds.
- Nitrogen (N): Nitrogen’s configuration is 1s2 2s2 2p3, allowing it to form three covalent bonds, as seen in ammonia (NH3).
- Oxygen (O): With a configuration of 1s2 2s2 2p4, oxygen can form two covalent bonds, which is crucial in water (H2O).
Understanding Orbital Diagrams
Orbital diagrams are visual representations of the arrangement of electrons within an atom’s orbitals. They provide a clearer understanding of how electrons are distributed and how they may interact during chemical reactions.
How to Draw Orbital Diagrams for Carbon
To draw the orbital diagram for carbon, individuals should follow these steps:
- Draw boxes to represent each orbital level (1s, 2s, 2p).
- Place arrows in the boxes to represent electrons. Each box can hold a maximum of two electrons, represented by arrows pointing in opposite directions.
- Follow Hund’s rule for the 2p orbitals, filling empty orbitals before pairing the electrons.
The resulting diagram shows two electrons in the 1s box, two in the 2s box, and two arrows in the 2p boxes representing carbon’s electron configuration.
Significance of Orbital Hybridization
Orbital hybridization is a concept that explains how atomic orbitals mix to form new hybrid orbitals. In the case of carbon, its four valence electrons lead to the formation of four equivalent sp3 hybrid orbitals, allowing carbon to achieve tetrahedral geometry during bonding, such as in methane (CH4).
The process of hybridization contributes to carbon’s versatility and ability to form diverse structures, including chains, branches, and rings which are integral to organic molecules.
Common Misconceptions About Carbon’s Electron Arrangement
Many learners face misconceptions regarding carbon’s electron configuration, often assuming that all carbon atoms possess identical configurations regardless of bonding or charge. It’s essential to clarify that:
- Ionized forms of carbon, such as C+1 or C-1, will adjust electron configurations accordingly, losing or gaining electrons.
- The concept of excited states can also alter electron placement within orbitals, which is pertinent when discussing chemical reactions and bonding scenarios.
Applications of Electron Configuration in Science
Understanding electron configuration extends beyond the theoretical realm, having numerous practical applications in science and technology.
How Electron Configuration Influences Chemical Properties
The electron configuration of an atom governs how it reacts chemically. Elements with similar configurations tend to exhibit comparable chemical behaviors. For instance, groups of the periodic table display trends in reactivity due to their electron arrangements. Alkali metals, having a single electron in their outer orbital, are more reactive than noble gases, which have a full set of electrons making them chemically inert.
The Role of Carbon in Organic Chemistry
Carbon’s electron configuration allows for a wider variety of chemical bonds and functional groups. The versatility of carbon-based compounds is unparalleled, making it fundamental in organic chemistry. Carbon’s tetravalency facilitates the formation of complex molecules, leading to structural diversity in biological systems, pharmaceuticals, and materials science. The foundational building blocks of life, such as carbohydrates, proteins, lipids, and nucleic acids, all involve carbon.
Electron Configuration and Material Science
In materials science, understanding the electron configuration helps in designing materials with specific properties. The arrangement of electrons can influence the conductivity, reactivity, and thermal properties of materials. For example, carbon allotropes like graphite and diamond exhibit distinct physical properties due to differences in their atomic arrangements and bonding configurations, stemming from their electron configurations.
Conclusion and Further Reading
Summary of Key Takeaways
The electron configuration of carbon, particularly represented as 1s2 2s2 2p2, plays a critical role in its chemical behavior, forming the basis for countless organic compounds. Understanding carbon’s electron arrangement and its interaction through hybridization provides insight into the molecular architectures that define biological and synthetic processes.
Recommended Resources for Further Study
- Chemistry LibreTexts on Electron Configurations
- Detailed Electron Configuration for Carbon
- YouTube Tutorial on Carbon Electron Configuration
Future Trends in Electron Configuration Research
The future of electron configuration research lies in its application to emerging technologies, including quantum computing and nanotechnology. As we deepen our understanding of electron behavior and configurations, the ability to manipulate electronic properties at the atomic level will lead to innovations in materials design, energy solutions, and synthetic biology.